Blood-based Biomarker for Sports Medicine Market
Blood-based Biomarker for Sports Medicine Market Size and Share Forecast Outlook 2025 to 2035
Blood-based biomarker for sports medicine market is projected to grow from USD 0.9 billion in 2025 to USD 1.8 billion by 2035, at a CAGR of 7.3%. CK will dominate with a 18.1% market share, while will lead the segment with a 0.0% share.
Blood-based Biomarker for Sports Medicine Market Forecast and Outlook 2025 to 2035
The global blood-based biomarker for sports medicine market is projected to reach USD 1.76 billion by 2035, recording an absolute increase of USD 0.89 billion over the forecast period. The market is valued at USD 0.87 billion in 2025 and is set to rise at a CAGR of 7.3% during the assessment period.
Quick Stats for Blood-based Biomarker for Sports Medicine Market
- Blood-based Biomarker for Sports Medicine Market Value (2025): USD 0.87 billion
- Blood-based Biomarker for Sports Medicine Market Forecast Value (2035): USD 1.76 billion
- Blood-based Biomarker for Sports Medicine Market Forecast CAGR: 7.3%
- Leading Type in Blood-based Biomarker for Sports Medicine Market: CK
- Key Growth Regions in Blood-based Biomarker for Sports Medicine Market: North America, Europe, and Asia Pacific
- Top Players in Blood-based Biomarker for Sports Medicine Market: Abbott, BIOMÉRIEUX, F. Hoffmann-La Roche Ltd., ARUP Laboratories, Siemens Healthineers AG, RayBiotech, Inc, Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc, Beckman Coulter, Inc., Randox Laboratories Ltd.
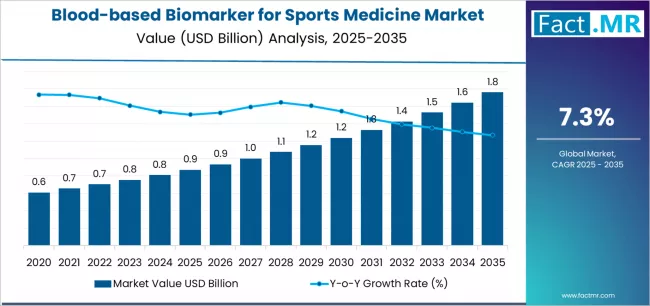
The overall market size is expected to grow by approximately 2.0 times during the same period, supported by increasing sports participation rates and rising awareness of injury prevention worldwide, driving demand for diagnostic biomarker testing and increasing investments in sports medicine research and athlete health monitoring technologies globally.
The sports medicine and athletic healthcare sectors face mounting pressure to prevent performance-limiting injuries while meeting evolving demands for rapid recovery protocols, with modern blood-based biomarker testing providing documented injury assessment capabilities and recovery monitoring benefits compared to traditional clinical evaluation alternatives.
Rising professional sports investment and expanding sports science infrastructure across emerging economies create substantial opportunities for diagnostic manufacturers and sports medicine clinics. However, limited standardization of biomarker reference ranges and reimbursement challenges for preventive testing may pose obstacles to widespread clinical adoption.
The CK segment dominates market activity with an 18.1% share in 2025, driven by the extensive athletic population requiring proven muscle damage assessment and training load monitoring capabilities across professional and amateur sports applications worldwide.
Sports medicine practitioners increasingly recognize the practical benefits of creatine kinase testing, with typical assay applications providing effective muscle breakdown detection and overtraining assessment at competitive price points through established laboratory distribution networks. The CRP segment maintains substantial market presence with USD 0.14 billion in 2025, supported by inflammation monitoring and injury severity assessment driving preference for C-reactive protein measurement in post-injury recovery protocols.
Professional sports emerge as a critical application category, reflecting institutional emphasis on injury prevention and performance optimization capabilities. Clinical laboratory applications represent significant testing volume, driven by comprehensive biomarker panel requirements and point-of-care testing integration for athlete health management across diverse sports disciplines.
North America maintains market leadership with a 44.0% share in 2025 and USD 0.38 billion in 2025, supported by high sports participation rates and advanced sports medicine infrastructure across USA and Canadian athletic programs.
Europe demonstrates strong presence with USD 0.22 billion in 2025 driven by professional football integration and clinical research excellence, while Asia Pacific shows USD 0.14 billion with fastest growth trajectory through expanding youth sports programs and injury awareness.
USA leads country-level growth at a 7.6% CAGR through extensive research partnerships and professional sports adoption, followed by India at 8.0% supported by rapid youth sports injury incidence and healthcare modernization.
The competitive landscape features moderate concentration with Abbott holding 15.0% market share, while established players including BIOMÉRIEUX, F. Hoffmann-La Roche Ltd., and Siemens Healthineers AG compete through comprehensive diagnostic portfolios and sports medicine testing capabilities across diverse athletic applications.
Blood-based Biomarker for Sports Medicine Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the blood-based biomarker for sports medicine market is projected to expand from USD 0.87 billion to USD 1,150.6 million, resulting in a value increase of USD 283.5 million, which represents 31.7% of the total forecast growth for the period. This phase of development will be shaped by rising demand for injury prevention screening in professional sports organizations, product innovation in point-of-care testing platforms with rapid turnaround capabilities, as well as expanding integration with wearable technology and digital health monitoring systems. Companies are establishing competitive positions through investment in sports medicine research facilities, advanced biomarker assay development, and strategic market expansion across professional athletics, collegiate programs, and sports medicine clinic applications.
From 2029 to 2035, the market is forecast to grow from USD 1,150.6 million to USD 1.76 billion, adding another USD 610.2 million, which constitutes 68.3% of the overall expansion. This period is expected to be characterized by the expansion of specialized biomarker applications, including multi-marker panels for comprehensive athlete health assessment and recovery optimization algorithms tailored for specific injury types, strategic collaborations between diagnostic companies and professional sports leagues, and an enhanced focus on evidence-based protocols and clinical validation studies. The growing emphasis on youth sports injury prevention and rising adoption of personalized training programs based on biomarker feedback will drive demand for comprehensive blood-based biomarker testing solutions across diverse athletic populations.
Blood-based Biomarker for Sports Medicine Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 0.87 billion |
| Market Forecast Value (2035) | USD 1.76 billion |
| Forecast CAGR (2025-2035) | 7.3% |
Why is the Blood-based Biomarker for Sports Medicine Market Growing?
The blood-based biomarker for sports medicine market grows by enabling sports medicine professionals and athletic trainers to assess injury risk while accessing objective physiological data without substantial imaging complexity requirements. Sports organizations and healthcare providers face mounting pressure to prevent career-threatening injuries and optimize recovery protocols while managing diverse athlete health needs across professional and amateur levels, with modern blood-based biomarker testing typically providing superior early detection and monitoring capabilities compared to symptom-based assessment alternatives, making diagnostic adoption essential for performance-oriented athletic positioning. The sports medicine industry's need for quantitative injury assessment and individualized training load management creates demand for comprehensive biomarker solutions that can provide superior muscle damage detection, maintain inflammation tracking accuracy, and ensure reliable recovery monitoring without compromising athlete availability or training continuity standards.
Professional sports initiatives promoting player safety and injury reduction protocols drive adoption in team environments, training facilities, and sports medicine clinics, where biomarker performance has a direct impact on injury prevention and career longevity. The increasing professionalization of youth sports has created expanding requirements for health monitoring systems that protect developing athletes, supporting sustained demand for preventive biomarker testing across all competitive levels. Rising healthcare expenditure in sports organizations enables greater investment in diagnostic technologies with proven injury prediction and recovery optimization capabilities. However, cost constraints among amateur athletic programs and the complexity of biomarker interpretation without standardized reference ranges may limit accessibility of comprehensive multi-marker panels among developing sports markets with limited medical resources for sophisticated athlete monitoring solutions.
Segmental Analysis
The market is segmented by type and region. By type, the market is divided into CK, myoglobin, lactate, WBC, urea, CRP, and others. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
By Type, Which Segment Accounts for the Dominant Market Share?

The CK segment represents the dominant force in the blood-based biomarker for sports medicine market, capturing approximately 18.1% of total market share in 2025 with USD 157.1 million in 2025. This category encompasses solutions featuring proven muscle damage detection and overtraining assessment capabilities, including total creatine kinase and CK-MB isoenzyme measurements that enable superior exercise-induced muscle injury monitoring and training load optimization across all professional and collegiate athletic applications.
The CK segment's market leadership stems from its superior clinical utility advantages, with assays capable of addressing diverse sports medicine needs while maintaining consistent analytical performance and broad laboratory accessibility across all healthcare settings.
The CRP segment maintains substantial market presence with USD 0.14 billion in 2025, serving sports medicine practitioners and athletic trainers who require inflammation monitoring with systemic injury assessment properties for post-trauma recovery tracking and chronic overuse condition evaluation.
These assays offer effective inflammatory status evaluation for athlete populations while providing sufficient sensitivity to meet contemporary sports medicine diagnostic demands. The CRP segment demonstrates consistent adoption, driven by expanding awareness of inflammation's role in injury recovery and increasing emphasis on objective recovery metrics.
Within the CK segment, monitoring applications in endurance sports demonstrate particularly strong adoption, driven by coach preference for objective training load assessment that prevents overtraining syndrome through serial CK measurement. This sub-segment benefits from established protocols in cycling, marathon running, and triathlon training programs requiring muscle damage surveillance.
Key technological advantages driving the CK segment include:
- Advanced analytical sensitivity with wide measurement ranges that enhance muscle damage detection and ensure consistent performance monitoring across diverse athletic populations and injury severities
- Established clinical interpretation allowing reliable assessment across different sports disciplines without extensive validation complexity or sport-specific reference development
- Enhanced turnaround characteristics enabling rapid testing and same-day results while maintaining assay precision and clinical decision-making utility
- Superior evidence base providing optimal clinical validation for various sports medicine applications and injury prevention protocols without extensive research requirements
What is the Overview of the Other Tye Segments?
Myoglobin represents a significant biomarker category with USD 104.0 million in 2025, accounting for approximately 18% of the total market, demonstrating steady demand through requirements for acute muscle injury assessment and early detection of severe rhabdomyolysis in extreme training conditions. This segment benefits from rapid release kinetics following muscle damage and established use in emergency medicine settings adapted for sports applications.
The lactate segment with USD 95.0 million in 2025 holds around 17% market share, serving sports scientists requiring metabolic assessment and training intensity optimization through lactate threshold testing. These measurements offer effective aerobic capacity evaluation and individualized training zone determination for endurance athlete populations.
The WBC category with USD 86.7 million captures about 15% of the market, including white blood cell monitoring for immune function assessment and infection risk evaluation, while urea with USD 65.0 million represents roughly 12% market share, addressing hydration status and protein metabolism monitoring.
The others segment with USD 215.1 million accounts for the largest share at approximately 38%, encompassing emerging biomarkers including troponin, cortisol, testosterone, and specialized inflammatory markers serving advanced sports medicine applications.
Key biomarker segment dynamics include:
- CK adoption patterns accelerating across team sports with emphasis on training load management and injury prevention in professional organizations
- CRP utilization requirements driving demand for inflammation monitoring and recovery assessment in post-injury rehabilitation programs
- Integration of multi-marker panels enabling comprehensive athlete health assessment through combined muscle damage, inflammation, and metabolic markers
- Growing emphasis on point-of-care testing driving rapid biomarker platforms without traditional central laboratory turnaround limitations
What are the Drivers, Restraints, and Key Trends of the Blood-based Biomarker for Sports Medicine Market?
The market is driven by three concrete demand factors tied to athletic outcomes. First, increasing sports injury incidence creates expanding demand for objective injury assessment biomarkers, with muscle and tendon injuries representing critical concerns affecting athletic performance worldwide, requiring comprehensive diagnostic availability. Second, professionalization of youth and collegiate sports drives increased adoption of preventive health monitoring, with many sports organizations implementing systematic biomarker screening and athlete health surveillance programs by 2030. Third, technological advancements in point-of-care testing platforms enable more accessible and rapid biomarker measurement that improves clinical decision-making while reducing laboratory turnaround times and enabling field-based testing capabilities.
Market restraints include limited standardization of reference ranges and sport-specific normative values that can challenge clinicians in interpreting biomarker results, particularly when athletes present with baseline values outside population norms and inter-individual variability proves substantial. Reimbursement limitations for preventive screening and performance monitoring applications pose another significant challenge, as blood-based biomarker testing for sports medicine often lacks insurance coverage in non-clinical settings, potentially affecting adoption rates and limiting accessibility. Technical complexity regarding biomarker kinetics and result interpretation creates additional barriers for non-specialist practitioners, demanding extensive education initiatives and clinical pathway development that increase implementation costs.
Key trends indicate accelerated multi-marker panel adoption in professional sports, particularly North America and Europe, where team physicians demonstrate commitment to comprehensive athlete health assessment through combined muscle damage, inflammation, and recovery markers. Point-of-care technology penetration trends toward sideline testing capabilities with immediate results enable real-time clinical decisions that optimize return-to-play protocols and training adjustments. However, the market thesis could face disruption if significant advances in non-invasive monitoring technologies or major shifts in sports medicine practice toward imaging-based assessment reduce reliance on traditional blood-based biomarker testing methodologies.
Analysis of the Blood-based Biomarker for Sports Medicine Market by Key Countries
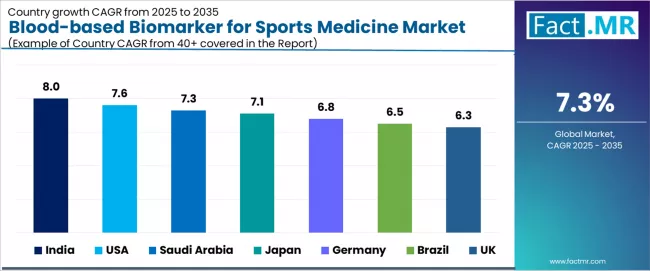
| Country | CAGR (2025-2035) |
|---|---|
| India | 8.0% |
| USA | 7.6% |
| Saudi Arabia | 7.3% |
| Japan | 7.1% |
| Germany | 6.8% |
| Brazil | 6.5% |
| UK | 6.3% |
The global blood-based biomarker for sports medicine market is expanding steadily, with India leading at an 8.0% CAGR through 2035, driven by rapid rise in youth sports injuries, expanding sports participation rates, and growing awareness of preventive health monitoring supporting diagnostic testing adoption. USA follows at 7.6%, supported by high sports participation rates, extensive research and development partnerships, and professional sports organization integration.
Saudi Arabia records 7.3%, reflecting emerging landscape with football-driven demand and government sports program funding. Japan grows at 7.1%, anchored by increasing collegiate sports injuries and advanced healthcare infrastructure. Germany advances at 6.8%, leveraging clinical research excellence and sports medicine facility development. Brazil posts 6.5%, focusing on government sports infrastructure funding, while UK grows steadily at 6.3%, emphasizing rising sports-related injury incidence and professional sports adoption.
How is the USA Leading Research-Driven Market Development?
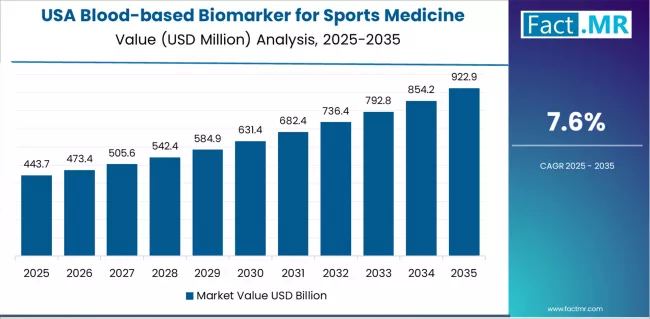
USA demonstrates strong growth potential in the blood-based biomarker for sports medicine market with a CAGR of 7.6% through 2035. The country's leadership position stems from high sports participation rates across professional, collegiate, and youth levels, comprehensive sports medicine infrastructure, and extensive research partnerships driving biomarker validation studies.
Growth is concentrated in major metropolitan sports medicine centers and professional team facilities, including those in New York, Los Angeles, Boston, and Chicago, where sports medicine physicians utilize blood-based biomarkers for injury risk screening and recovery monitoring.
Distribution channels through specialized sports medicine laboratories and point-of-care testing providers expand biomarker accessibility across athletic training facilities and team medical departments. The country's robust research funding provides strong momentum for biomarker validation, including comprehensive clinical trials and professional sports league partnerships.
Key market factors:
- Professional sports integration concentrated in major league organizations with advanced sports science departments and injury prevention programs
- Research partnerships through academic medical centers and sports medicine institutes enabling biomarker validation and protocol development
- Comprehensive healthcare infrastructure ecosystem, including specialized laboratories with sports medicine testing expertise and rapid turnaround capabilities
- Innovation leadership featuring companies like Abbott offering point-of-care platforms for field-based testing applications
Why is India Emerging as a High-Growth Market?
In major urban centers including Mumbai, Delhi, Bangalore, and Chennai, the adoption of blood-based biomarker testing is accelerating across sports medicine clinics and athletic training facilities, driven by rapid increase in youth sports participation and growing awareness of injury prevention. The market demonstrates strong growth momentum with a CAGR of 8.0% through 2035, linked to comprehensive sports infrastructure development and increasing focus on athlete health management.
Indian sports medicine practitioners are implementing biomarker screening protocols and adopting injury prevention strategies to protect young athletes while meeting growing expectations in sports safety standards. The country's expanding middle class creates ongoing demand for organized youth sports programs requiring health monitoring, while increasing emphasis on professional cricket and Olympic sports drives adoption of advanced sports medicine diagnostics.
Key development areas:
- Sports medicine clinics and athletic training centers leading blood-based biomarker adoption with emphasis on injury prevention and athlete health monitoring
- Healthcare infrastructure expansion through private hospital sports medicine departments and specialized athletic performance centers
- Government initiatives enabling improved sports medicine education and athletic healthcare standards supporting biomarker testing awareness
- Growing preference for preventive screening alongside reactive injury assessment offering comprehensive athlete health management
What Drives Japan's Advanced Sports Medicine Integration?
Japan market expansion is driven by comprehensive collegiate sports programs, advanced healthcare infrastructure, and established sports medicine specialization supporting reliable biomarker testing adoption. The country demonstrates steady growth potential with a CAGR of 7.1% through 2035, supported by increasing sports injury incidence in school athletics and professional baseball organization requirements.
Japanese sports medicine practitioners face clinical challenges related to reference range establishment for Asian athletic populations, requiring biomarker manufacturers to provide population-specific validation data and interpretation guidelines. However, established university sports programs and growing professional sports investment create stable baseline demand for blood-based biomarkers, particularly in collegiate athletics where injury prevention drives primary testing implementation.
Market characteristics:
- Collegiate athletics and professional baseball segments showing robust demand with substantial annual testing volumes across diverse sports applications
- Regional sports medicine infrastructure varying between university hospital centers emphasizing research integration and private clinics focusing on athlete services
- Future projections indicate continued technology adoption with emphasis on point-of-care platforms and multi-marker panel development
- Growing emphasis on youth sports safety and injury prevention supporting biomarker screening integration and systematic monitoring protocols
How Does Germany Demonstrate Clinical Research Leadership?
The market in Germany leads in sports medicine research based on integration with advanced clinical research facilities and comprehensive sports science programs supporting biomarker validation studies. The country shows strong potential with a CAGR of 6.8% through 2035, driven by professional football requirements and sports medicine excellence in major markets, including Bavaria, North Rhine-Westphalia, Baden-Württemberg, and Hesse.
German sports medicine physicians are adopting evidence-based biomarker protocols and specialized testing panels for professional athlete monitoring, particularly in Bundesliga football teams requiring comprehensive injury prevention and recovery optimization. Distribution channels through university sports medicine departments and specialized laboratories expand coverage across professional sports organizations and Olympic training centers.
Leading market segments:
- Professional football teams in major cities implementing comprehensive biomarker monitoring with emphasis on injury prevention and performance optimization
- Research partnerships between universities and sports organizations achieving clinical validation of biomarker applications
- Strategic collaborations between diagnostic companies and sports medicine institutes expanding evidence generation and protocol development
- Focus on standardized testing protocols and reference range establishment addressing clinical interpretation requirements and athletic population specificity
What Positions Saudi Arabia for Sports Medicine Investment?
In major cities including Riyadh, Jeddah, Dammam, and Mecca, sports medicine facilities are implementing blood-based biomarker testing featuring professional football integration and youth sports program expansion, with documented implementation showing substantial health monitoring improvement through systematic screening and injury prevention initiatives. The market shows strong growth potential with a CAGR of 7.3% through 2035, linked to government sports funding, football-driven healthcare demand, and emerging professional sports league development in major regions.
Sports medicine providers are adopting international testing standards and implementing biomarker-based injury prevention to enhance athlete safety while maintaining performance standards demanded by competitive sports environments. The country's sports sector investment creates ongoing opportunities for diagnostic companies establishing local partnerships that address regional sports medicine requirements.
Market development factors:
- Professional football clubs and national sports programs leading blood-based biomarker adoption across Saudi Arabia
- Sports medicine infrastructure development providing growth opportunities in both government sports facilities and private athletic training centers
- Strategic partnerships between international diagnostic companies and local healthcare providers expanding technology transfer
- Emphasis on youth sports safety and athlete health management supporting biomarker testing awareness and adoption
How does the UK Show Professional Sports Integration?
In major metropolitan areas including London, Manchester, Birmingham, and Liverpool, professional sports organizations are implementing blood-based biomarker testing featuring Premier League football integration and Olympic athlete monitoring, with documented programs showing substantial injury prevention improvement through systematic health surveillance and evidence-based protocols.
The market shows steady growth potential with a CAGR of 6.3% through 2035, linked to rising sports injury incidence, professional sports medicine development, and emerging injury prevention awareness in major regions. UK sports medicine practitioners are adopting validated biomarker panels and implementing standardized screening to enhance athlete safety while maintaining evidence standards demanded by professional sports governance structures. The country's established sports medicine infrastructure creates ongoing opportunities for diagnostic manufacturers establishing professional sports partnerships that address elite athlete requirements.
Market development factors:
- Professional football clubs and Olympic programs leading adoption of blood-based biomarkers across UK
- Sports injury awareness providing growth opportunities in both professional sports and collegiate athletics applications
- Strategic partnerships between diagnostic companies and sports governing bodies expanding standardized testing protocols
- Emphasis on evidence-based sports medicine and clinical validation addressing healthcare quality standards and professional practice guidelines
How Does Brazil Show Government Sports Investment?
Brazil's blood-based biomarker for sports medicine market demonstrates comprehensive government funding focused on sports infrastructure development and Olympic program support, with documented integration of biomarker testing achieving substantial improvement in athlete health management across national training centers.
The country maintains steady growth momentum with a CAGR of 6.5% through 2035, driven by government sports investment initiatives emphasizing athlete safety and football culture integration aligned with Brazilian sports traditions. Major metropolitan areas, including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, showcase expanding adoption of biomarker testing where sports medicine programs integrate with national athletic development initiatives and comprehensive youth sports systems.
Key market characteristics:
- National sports programs and football academies driving demand for blood-based biomarkers with emphasis on youth athlete protection and talent development
- Government sports partnerships enabling consistent sports medicine advancement with comprehensive training center development programs
- Infrastructure collaboration between Ministry of Sports initiatives and international diagnostic companies expanding testing accessibility
- Emphasis on injury prevention and athlete longevity addressing sports development requirements and international competition preparation
What Characterizes India's Youth Sports Expansion?
In major metropolitan centers including Mumbai, Delhi, Kolkata, and Bangalore, the adoption of blood-based biomarker testing is expanding across youth sports programs and cricket academies, driven by organized sports growth and parental awareness of injury prevention. The market demonstrates strong growth momentum with a CAGR of 8.0% through 2035, linked to youth sports participation expansion and increasing focus on athlete health protection.
Indian sports medicine providers are implementing preventive screening programs and adopting biomarker monitoring to protect young athletes while addressing growing concerns about sports injury incidence in competitive youth environments. The country's expanding sports culture creates ongoing demand for injury prevention solutions, while increasing healthcare awareness drives adoption of objective health monitoring approaches.
Key development areas:
- Cricket academies and youth sports programs leading blood-based biomarker adoption with emphasis on injury prevention and health screening
- Private sports medicine infrastructure development through specialized clinics and athletic training facilities supporting testing availability
- Sports organization initiatives enabling improved athlete health standards and systematic monitoring protocols
- Integration of preventive healthcare and sports participation supporting biomarker awareness and systematic screening adoption
Europe Market Split by Country
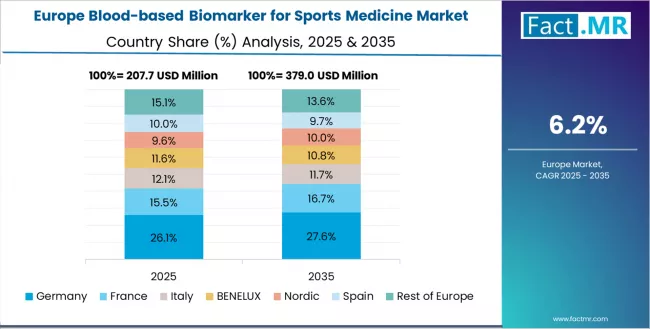
The blood-based biomarker for sports medicine market in Europe is projected to grow steadily over the forecast period, driven by professional sports integration, advanced sports medicine infrastructure, and comprehensive clinical research capabilities supporting biomarker validation and adoption. In 2025, the regional distribution reflects varied levels of adoption across major European markets, with Germany, the UK, France, Italy, Spain, and the Rest of Europe contributing distinct shares to overall demand.
Germany is expected to maintain a strong leadership position, accounting for an estimated 27% of the European market in 2025. This leadership is supported by extensive sports science research facilities, established professional football integration, and comprehensive university sports medicine programs serving major European markets. Consistent investment in diagnostics, biomarker validation, and elite athlete monitoring strengthens the country’s role as the region’s central adoption hub.
The UK follows with meaningful market presence and is projected to capture approximately 18% of Europe’s 2025 demand. Premier League football requirements, Olympic sports programs, and expanding evidence-based biomarker protocols across elite athlete populations support this share. The country’s strong diagnostics ecosystem and performance-science orientation contribute to sustained biomarker integration across training, recovery, and athlete-health assessment frameworks.
France holds a substantial market share, estimated at 16% in 2025, supported by professional sports development and national athletic training center integration. Broad adoption across football, rugby, and Olympic sports, combined with investments in physiological assessment and sports medicine research, reinforces its sizeable contribution. France’s growing preference for advanced biomarker-based monitoring also supports continued uptake across institutional and professional sports environments.
Italy commands an estimated 12% share of the European market in 2025. Strong participation in professional sports, combined with rising modernization initiatives within national sports medicine institutes, supports increasing demand for biomarker-based athlete monitoring. Italy’s expanding football and cycling ecosystems also contribute to sustained biomarker adoption in performance evaluation and injury-reduction programs.
Spain accounts for an estimated 9% of Europe’s 2025 market, driven by growing adoption in football academies, elite development programs, and professional sports organizations. Leading football clubs continue to expand performance analytics and physiological monitoring infrastructures, contributing to heightened interest in blood-based biomarkers as part of athlete conditioning, readiness testing, and return-to-play workflows.
The Rest of Europe region is anticipated to gain momentum and represents an estimated 18% of the 2025 market. Increasing blood-based biomarker adoption in Nordic countries, supported by strong sports medicine research capacity, combines with modernizing sports science systems in emerging Eastern European markets. Expanding athlete-health monitoring development and institutional investment across these regions reinforces the growing contribution of the Rest of Europe segment.
How Does Evidence-Based Medicine Define Blood-based Biomarker Adoption in Japan?
The Japanese blood-based biomarker for sports medicine market demonstrates a mature and research-focused landscape, characterized by sophisticated integration of validated testing protocols with advanced sports medicine programs across collegiate athletics, professional baseball, and Olympic training facilities. Japan's emphasis on scientific rigor and evidence-based practice drives demand for clinically validated biomarker solutions that support comprehensive injury prevention initiatives and systematic health monitoring in competitive athletics.
The market benefits from strong partnerships between international diagnostic companies and domestic sports medicine centers, including university hospital sports departments and professional team medical facilities, creating comprehensive testing ecosystems that prioritize data quality and clinical interpretation programs. Collegiate sports segments showcase advanced biomarker implementations where systematic monitoring achieves performance improvements through integrated injury prevention systems and comprehensive athlete health management protocols.
What Drives Professional Sports Focus in South Korea's Market?
The South Korean blood-based biomarker for sports medicine market is characterized by strong professional sports integration, with companies maintaining significant presence through comprehensive athlete health programs in professional baseball, football, and esports organizations requiring systematic health monitoring.
The market is demonstrating a growing emphasis on preventive sports medicine and specialized biomarker capabilities, as Korean sports organizations increasingly demand evidence-based injury prevention that combines objective biomarker data with performance optimization deployed across professional team facilities and national training centers.
Local sports medicine providers and diagnostic distributors are maintaining market presence through strategic emphasis on professional sports relationships, offering comprehensive testing services including rapid turnaround and specialized interpretation for elite athlete populations. The competitive landscape shows increasing collaboration between diagnostic companies and sports leagues, creating integrated health monitoring models that combine biomarker testing with performance analytics and injury prevention optimization capabilities.
Competitive Landscape of the Blood-based Biomarker for Sports Medicine Market
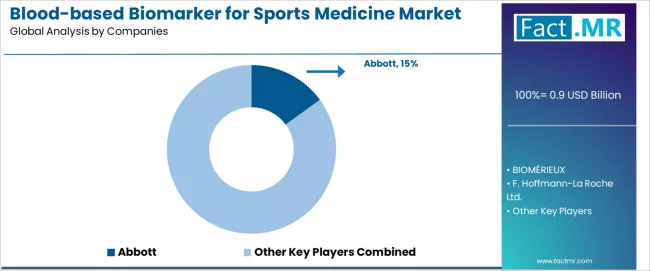
The blood-based biomarker for sports medicine market features approximately 15-20 meaningful players with moderate concentration, where the top three companies control roughly 30-35% of global market share through established diagnostic portfolios and extensive sports medicine partnerships. Competition centers on clinical validation, point-of-care capabilities, and sports medicine expertise rather than price competition alone.
Market leaders include Abbott, BIOMÉRIEUX, and F. Hoffmann-La Roche Ltd., which maintain competitive advantages through comprehensive diagnostic assay portfolios, global laboratory networks, and deep expertise in the clinical diagnostics sector, creating high customer loyalty among sports medicine practitioners and athletic training professionals.
These companies leverage established healthcare relationships and ongoing research collaborations to defend market positions while expanding into adjacent categories including wearable technology integration and digital health monitoring platforms.
Challengers encompass Siemens Healthineers AG, Thermo Fisher Scientific, Inc., and Bio-Rad Laboratories, Inc, which compete through comprehensive laboratory solutions and strong market presence in key clinical diagnostics and research territories. Specialized diagnostics companies, including ARUP Laboratories, RayBiotech, Inc, and Randox Laboratories Ltd., focus on specific biomarker panels or testing platforms, offering differentiated capabilities in sports medicine-focused assays, multi-marker testing, and point-of-care solutions.
Regional players and emerging sports medicine diagnostics companies create competitive pressure through specialized testing panels and sports-specific interpretation services, particularly in high-growth markets including India and China, where local laboratory capabilities provide advantages in market access and practitioner relationships.
Market dynamics favor companies that combine established diagnostic technologies with sports medicine clinical expertise that addresses the complete value chain from biomarker measurement through clinical interpretation and athletic health management programs.
Strategic emphasis on point-of-care development, multi-marker validation, and professional sports partnerships enables differentiation in increasingly evidence-focused sports medicine markets across developed and emerging economies.
Global Blood-based Biomarker for Sports Medicine Market - Stakeholder Contribution Framework
Blood-based biomarker solutions represent a critical sports medicine diagnostic technology that enables athletic trainers, team physicians, and sports medicine clinics to assess injury risk and monitor recovery without complex imaging requirements, typically providing enhanced objective assessment and early detection capabilities compared to symptom-based evaluation alternatives while ensuring improved clinical decision-making and consistent athlete monitoring.
With the market projected to grow from USD 0.87 billion in 2025 to USD 1.76 billion by 2035 at a 7.3% CAGR, these solutions offer compelling advantages - superior injury detection, enhanced recovery monitoring, and objective training load assessment - making them essential for professional sports applications, CK biomarker testing (18.1% type share in 2025), and diverse athletic populations requiring reliable health monitoring solutions. Scaling market penetration and clinical adoption requires coordinated action across sports medicine research, clinical validation studies, diagnostic manufacturers, sports organizations, and healthcare provider education initiatives.
How Could Governments Spur Sports Medicine Development and Athlete Safety?
- Sports Safety Programs: Include injury prevention in national athletic health initiatives, providing targeted support for biomarker screening in youth sports programs and supporting sports medicine facilities through research grants and infrastructure assistance.
- Healthcare Policy & Research Support: Implement coverage frameworks for preventive sports medicine testing in national health systems, provide research incentives for companies developing validated biomarker protocols and athlete-specific reference ranges, and establish favorable regulatory frameworks that encourage sports medicine innovation.
- Clinical Standards Development: Create standardized testing protocols for blood-based biomarkers in sports medicine across professional, collegiate, and youth applications, establish clear interpretation guidelines and sport-specific reference values for practitioner guidance, and develop international harmonization frameworks that facilitate evidence sharing.
- Sports Medicine Education: Fund specialized training programs for athletic trainers, sports medicine physicians, and exercise physiologists. Invest in continuing education initiatives that bridge biomarker science with clinical sports medicine practice and athlete health management protocols.
- Athlete Protection Policies: Establish mandatory health screening requirements for youth sports organizations, support injury prevention research through funding programs, and create regulatory environments that encourage systematic athlete health monitoring.
How Could Sports Medicine Organizations Support Market Development?
- Clinical Guidelines & Protocols: Define standardized biomarker testing protocols for injury risk assessment, recovery monitoring, and return-to-play decisions, establish universal interpretation frameworks and sport-specific benchmarks, and create certification programs for biomarker-based sports medicine practice.
- Practitioner Education & Best Practices: Lead educational initiatives about blood-based biomarker applications, emphasizing injury prevention benefits, recovery optimization capabilities, and evidence-based clinical decision-making compared to subjective assessment alternatives.
- Research Standards: Develop guidelines for biomarker validation studies, clinical trial design for sports medicine applications, and evidence quality assessment, ensuring rigorous evaluation across diagnostic development and clinical implementation programs.
- Professional Development: Run training programs for sports medicine physicians, athletic trainers, and strength coaches on optimizing biomarker utilization, clinical interpretation, and athlete health management in diverse sports environments.
How Could Diagnostic Manufacturers Strengthen the Ecosystem?
- Advanced Assay Development: Develop next-generation biomarker platforms with enhanced point-of-care capabilities, improved multi-marker panels, and sport-specific reference ranges that enhance clinical utility while maintaining optimal analytical performance and rapid turnaround characteristics.
- Clinical Validation Programs: Provide comprehensive validation studies, longitudinal athlete monitoring research with injury outcome data, and sport-specific evidence demonstrating clinical utility that supports sports medicine adoption and healthcare decision-making.
- Healthcare Provider Education: Offer comprehensive training resources about biomarker interpretation, optimal testing protocols, and clinical application guidelines that help sports medicine practitioners achieve superior athlete health outcomes aligned with evidence-based practice.
- Research & Partnership Networks: Build comprehensive sports medicine research collaborations, professional sports league partnerships, and clinical validation consortiums that ensure biomarker testing maintains high clinical standards and evidence quality across diverse athletic applications.
How Could Sports Organizations Navigate the Market?
- Comprehensive Health Monitoring: Expand biomarker screening programs across CK testing (18.1% dominance in 2025), inflammation markers, and metabolic assessments, with particular focus on injury prevention applications and recovery optimization for diverse athlete populations and competitive levels.
- Geographic Program Development: Establish biomarker monitoring in high-growth markets like India (8.0% CAGR) and USA (7.6% CAGR), while strengthening programs in established markets like Germany (6.8% CAGR) and Japan (7.1% CAGR) through optimized testing protocols and clinical partnerships.
- Evidence-Based Integration: Implement comprehensive outcome tracking systems combining biomarker data with injury incidence, return-to-play metrics, and performance outcomes that demonstrate program value and support continued investment.
- Athlete Education Programs: Develop educational initiatives explaining biomarker testing benefits, injury prevention strategies, and health monitoring importance addressing athlete engagement and compliance requirements.
How Could Investors and Financial Enablers Unlock Value?
- Technology Development Financing: Provide growth capital for established companies like Abbott, BIOMÉRIEUX, and F. Hoffmann-La Roche Ltd. to develop point-of-care platforms and multi-marker panels, particularly for sports medicine-specific applications with clinical validation requirements.
- Innovation Investment: Back diagnostic companies developing novel biomarkers, artificial intelligence-based interpretation algorithms, and integrated monitoring platforms that enhance clinical utility and athlete health management.
- Market Expansion Funding: Finance sports medicine research programs and professional sports partnership strategies for diagnostic companies establishing presence in high-growth regions, supporting clinical validation initiatives that accelerate adoption.
- Consolidation & Scale Opportunities: Support strategic acquisitions and market consolidation that create comprehensive sports medicine diagnostic portfolios, improve clinical evidence generation, and enhance competitive positioning against fragmented specialty providers across multiple sports applications.
Key Players in the Blood-based Biomarker for Sports Medicine Market
- Abbott
- BIOMÉRIEUX
- F. Hoffmann-La Roche Ltd.
- ARUP Laboratories
- Siemens Healthineers AG
- RayBiotech, Inc
- Thermo Fisher Scientific, Inc.
- Bio-Rad Laboratories, Inc
- Beckman Coulter, Inc.
- Randox Laboratories Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 0.87 billion |
| Type | CK, Myoglobin, Lactate, WBC, Urea, CRP, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | USA, India, Saudi Arabia, Japan, Germany, Brazil, UK, and 40+ countries |
| Key Companies Profiled | Abbott, BIOMÉRIEUX, F. Hoffmann-La Roche Ltd., ARUP Laboratories, Siemens Healthineers AG, RayBiotech, Inc, Thermo Fisher Scientific, Inc., Bio-Rad Laboratories, Inc, Beckman Coulter, Inc., Randox Laboratories Ltd. |
| Additional Attributes | Dollar sales by biomarker type categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with diagnostic manufacturers and sports medicine laboratories, clinical validation specifications and performance requirements, integration with sports medicine protocols and athlete health management systems, innovations in point-of-care testing and multi-marker platforms, and development of specialized applications with injury prevention and recovery optimization capabilities. |
Blood-based Biomarker for Sports Medicine Market by Segments
-
Type :
- CK
- Myoglobin
- Lactate
- WBC
- Urea
- CRP
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2025 to 2035
- CK
- Myoglobin
- Lactate
- WBC
- Urea
- CRP
- Others
- Y to o to Y Growth Trend Analysis By Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Type
- Competition Analysis
- Competition Deep Dive
- Abbott
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- BIOMÉRIEUX
- F. Hoffmann-La Roche Ltd.
- ARUP Laboratories
- Siemens Healthineers AG
- RayBiotech, Inc
- Thermo Fisher Scientific, Inc.
- Bio-Rad Laboratories, Inc
- Beckman Coulter, Inc.
- Randox Laboratories Ltd.
- Abbott
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 3: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 5: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: Latin America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 7: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Western Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 9: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Eastern Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 11: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: East Asia Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 13: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 15: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 16: Middle East & Africa Market Value (USD Million) Forecast by Type, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Type
- Figure 6: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Region
- Figure 9: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 10: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 11: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 12: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 17: North America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 18: North America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 19: North America Market Attractiveness Analysis by Type
- Figure 20: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 21: Latin America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 22: Latin America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 23: Latin America Market Attractiveness Analysis by Type
- Figure 24: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 25: Western Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 26: Western Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 27: Western Europe Market Attractiveness Analysis by Type
- Figure 28: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 29: Eastern Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 30: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 31: Eastern Europe Market Attractiveness Analysis by Type
- Figure 32: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: East Asia Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 34: East Asia Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 35: East Asia Market Attractiveness Analysis by Type
- Figure 36: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 37: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 38: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 39: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 40: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Middle East & Africa Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 42: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 43: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 44: Global Market - Tier Structure Analysis
- Figure 45: Global Market - Company Share Analysis
- FAQs -
How big is the blood-based biomarker for sports medicine market in 2025?
The global blood-based biomarker for sports medicine market is estimated to be valued at USD 0.9 billion in 2025.
What will be the size of blood-based biomarker for sports medicine market in 2035?
The market size for the blood-based biomarker for sports medicine market is projected to reach USD 1.8 billion by 2035.
How much will be the blood-based biomarker for sports medicine market growth between 2025 and 2035?
The blood-based biomarker for sports medicine market is expected to grow at a 7.3% CAGR between 2025 and 2035.
What are the key product types in the blood-based biomarker for sports medicine market?
The key product types in blood-based biomarker for sports medicine market are ck, myoglobin, lactate, wbc, urea, crp and others.
Which segment to contribute significant share in the blood-based biomarker for sports medicine market in 2025?
In terms of , segment to command 0.0% share in the blood-based biomarker for sports medicine market in 2025.