Controlled Release Drug Delivery Market
Controlled Release Drug Delivery Market Size and Share Forecast Outlook 2025 to 2035
Controlled release drug delivery market is projected to grow from USD 65.8 billion in 2025 to USD 181.2 billion by 2035, at a CAGR of 10.7%. Targeted Delivery will dominate with a 24.7% market share, while oral controlled drug delivery will lead the application segment with a 35.3% share.
Controlled Release Drug Delivery Market Forecast and Outlook (2025 to 2035
The global controlled release drug delivery market is projected to reach USD 181.2 billion by 2035, recording an absolute increase of USD 115.3 billion over the forecast period. The market is valued at USD 65.8 billion in 2025 and is set to rise at a CAGR of 10.7% during the assessment period.
Quick Stats for Controlled Release Drug Delivery Market (Updated on 7 November 2025)
- Controlled Release Drug Delivery Market Value (2025): USD 65.8 billion
- Controlled Release Drug Delivery Market Forecast Value (2035): USD 181.2 billion
- Controlled Release Drug Delivery Market Forecast CAGR: 10.7%
- Leading Technology in Controlled Release Drug Delivery Market: Targeted Delivery
- Key Growth Regions in Controlled Release Drug Delivery Market: Asia Pacific, North America, and Europe
- Top Players in Controlled Release Drug Delivery Market: Johnson & Johnson, Pfizer Inc., Merck & Co. Inc., AbbVie Inc., Novartis AG, Bayer AG, AstraZeneca plc, Corium International Inc., Alkermes plc, Amneal Pharmaceuticals
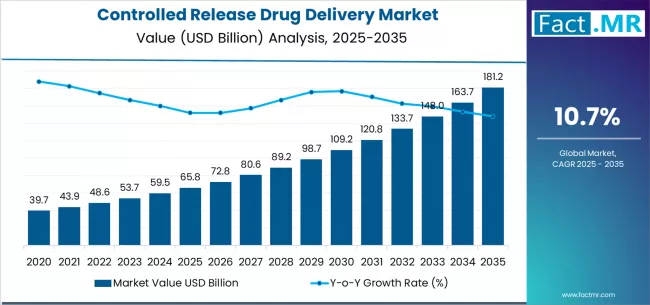
The overall market size is expected to grow by nearly 2.8 times during the same period, supported by increasing demand for patient-adherence optimization and advanced therapeutic delivery systems worldwide, driving demand for specialized pharmaceutical technologies and increasing investments in chronic disease management and personalized medicine initiatives globally.
The pharmaceutical industry faces mounting pressure to improve treatment outcomes while meeting stringent efficacy standards, with modern controlled release systems providing documented adherence improvement of 40-60% compared to conventional dosing regimens. Rising chronic disease prevalence and expanding biopharmaceutical development across emerging economies create substantial opportunities for drug delivery technology manufacturers and pharmaceutical service providers. However, high regulatory compliance requirements and technical challenges in formulation complexity may pose obstacles to market expansion.
The targeted delivery segment dominates market activity with approximately 24.7% share in 2025, driven by its superior therapeutic precision and capability to optimize drug concentration at specific sites for enhanced clinical outcomes across pharmaceutical applications worldwide. Healthcare professionals increasingly recognize the therapeutic benefits of targeted delivery systems, with typical applications ranging from oncology treatments to chronic disease management requiring controlled drug release.
The micro-encapsulation segment demonstrates the fastest growth potential with 11.8% CAGR, supported by advanced polymer technologies and versatility in protecting active pharmaceutical ingredients while enabling sustained release patterns. Oral controlled drug delivery emerges as the leading application category with 35.3% share, reflecting widespread adoption in chronic disease management, cardiovascular treatments, and neurological therapies across pharmaceutical development. Injectables represent the fastest-growing application segment at 12.2% CAGR, driven by long-acting formulation innovations and expanding biologic drug delivery demanding extended therapeutic windows.
Regional dynamics show North America maintaining market leadership with 42.6% share in 2024, supported by extensive research and development infrastructure and innovation capabilities across the USA market. Asia Pacific demonstrates the fastest growth trajectory at approximately 11.3% CAGR through 2035, driven by rapid pharmaceutical manufacturing expansion in China, India, and Southeast Asian markets.
Europe emphasizes precision medicine integration and regulatory advancement across Germany, UK, and France, while Latin America shows increasing adoption in Brazil's healthcare infrastructure. The competitive landscape features moderate concentration with Johnson & Johnson holding approximately 7.5% market share in 2024, while established players including Pfizer Inc., Merck & Co. Inc., and AbbVie Inc. compete through comprehensive product portfolios and advanced drug delivery capabilities across diverse therapeutic applications.
Controlled Release Drug Delivery Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the controlled release drug delivery market is projected to expand from USD 65.8 billion to USD 98.9 billion, resulting in a value increase of USD 33.1 billion, which represents 28.7% of the total forecast growth for the period. This phase of development will be shaped by rising demand for oral controlled delivery systems and injectable formulations in chronic disease management, product innovation in polymeric nanoparticle technologies and microsphere-based platforms, as well as expanding integration with biologics development and personalized medicine applications. Companies are establishing competitive positions through investment in specialized formulation capabilities, advanced polymer research, and strategic market expansion across oncology, neurology, and diabetes therapeutic areas.
From 2029 to 2035, the market is forecast to grow from USD 98.9 billion to USD 181.2 billion, adding another USD 82.3 billion, which constitutes 71.3% of the overall expansion. This period is expected to be characterized by the expansion of specialized delivery technologies, including advanced long-acting injectables and next-generation implantable systems tailored for specific therapeutic requirements, strategic collaborations between pharmaceutical companies and drug delivery specialists, and an enhanced focus on regulatory compliance and bioavailability optimization. The growing emphasis on patient adherence programs and precision medicine initiatives will drive demand for comprehensive controlled release solutions across diverse pharmaceutical applications.
Controlled Release Drug Delivery Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 65.8 billion |
| Market Forecast Value (2035) | USD 181.2 billion |
| Forecast CAGR (2025-2035) | 10.7% |
Why is the Controlled Release Drug Delivery Market Growing?
The controlled release drug delivery market grows by enabling pharmaceutical companies and healthcare providers to optimize therapeutic outcomes while accessing advanced delivery technologies without substantial in-house development requirements.
Pharmaceutical manufacturers and clinicians face mounting pressure to improve patient adherence and therapeutic efficacy while managing complex drug formulation requirements, with modern controlled release systems typically providing 40-60% adherence improvement compared to conventional multi-dose regimens, making delivery innovation essential for competitive clinical positioning.
The pharmaceutical industry's need for enhanced bioavailability and application-specific release mechanisms creates demand for comprehensive drug delivery solutions that can provide superior therapeutic control, maintain consistent plasma concentrations, and ensure reliable drug action without compromising patient convenience or treatment outcomes.
Government initiatives promoting chronic disease management and personalized medicine drive adoption in oncology treatments, diabetes care, and cardiovascular applications, where delivery system performance has a direct impact on treatment success and healthcare costs. The global shift toward biologics and complex molecules accelerates controlled release adoption, as these therapeutic agents require sophisticated delivery mechanisms to maintain stability and achieve optimal pharmacokinetics.
Growing emphasis on patient-centric care and reduced healthcare burden creates expanding opportunities for drug delivery manufacturers across both developed and emerging pharmaceutical markets. However, regulatory stringency constraints for extended-release approvals and the technical requirements for demonstrating bioequivalence with existing formulations may limit accessibility among smaller pharmaceutical companies and developing regions with limited resources for sophisticated drug delivery development.
Segmental Analysis
The market is segmented by technology, release mechanism, application, and region. By technology, the market is divided into targeted delivery, micro-encapsulation, transdermal & implants, Wurster & coacervation techniques, and others including liposomes & micro-electromechanical systems.
By application, the market is categorized into oral controlled drug delivery, injectable long-acting formulations, metered dose inhalers, transdermal & ocular patches, and drug-eluting stents & infusion pumps. By release mechanism, the market includes feedback-regulated systems and activated modulated delivery systems. Regionally, the market is divided into North America, Asia Pacific, Europe, Latin America, and Middle East & Africa.
Why is Targeted Drug Delivery Gaining Traction by Technology?
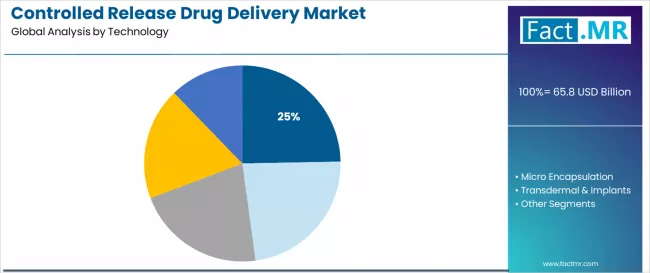
The targeted delivery segment represents the dominant force in the controlled release drug delivery market, capturing approximately 24.7% of total market share in 2025. This established technology category encompasses solutions featuring site-specific drug delivery and therapeutic optimization, including advanced ligand-receptor interactions and precision targeting mechanisms that enable superior clinical outcomes and reduced systemic side effects across all pharmaceutical applications.
The targeted delivery segment's market leadership stems from its superior therapeutic advantages, with solutions capable of addressing diverse disease requirements while maintaining consistent drug concentration at target sites and minimizing off-target effects across all treatment environments.
The micro-encapsulation segment demonstrates the fastest growth trajectory with a CAGR of 11.8% from 2025 to 2035, serving pharmaceutical developers who require advanced polymer matrix systems with enhanced active ingredient protection for sustained release applications. These solutions offer sophisticated encapsulation capabilities for diverse drug molecules while providing sufficient stability to meet contemporary regulatory demands.
The transdermal & implants segment maintains substantial market presence with 13.1% share, while Wurster & coacervation techniques hold 11.4% share in 2025. Other technologies including liposomes and micro-electromechanical systems collectively represent 35.6% market share, serving specialized therapeutic applications.
Key technological advantages driving the targeted delivery segment include:
- Advanced precision targeting with ligand-based delivery systems that enhance therapeutic efficacy and ensure site-specific drug accumulation
- Established clinical frameworks allowing streamlined drug development across different disease indications without extensive formulation modifications
- Enhanced bioavailability features enabling optimal therapeutic concentrations while maintaining patient safety and treatment tolerability
- Superior dose efficiency providing optimal clinical performance for various chronic and acute disease applications
What is the Outlook for Oral Controlled Release Drug Delivery Approaches?
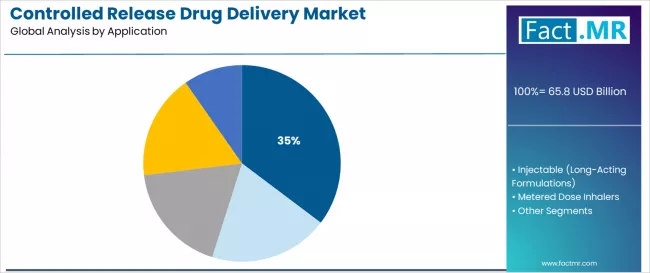
Oral controlled drug delivery dominates the controlled release drug delivery market with approximately 35.3% market share in 2025, reflecting the critical role of oral formulations in supporting patient convenience and therapeutic compliance worldwide. The oral controlled drug delivery segment's market leadership is reinforced by increasing chronic disease prevalence, patient preference for non-invasive administration, and rising needs for sustained therapeutic effect in pharmaceutical applications across developed and emerging markets.
The injectable long-acting formulations segment represents the fastest-growing application category, demonstrating a CAGR of 12.2% from 2025 to 2035 through specialized requirements for extended therapeutic windows, biologic drug delivery, and direct systemic administration capabilities. This segment benefits from growing biologics development demand that requires specific stability requirements, sustained plasma levels, and reduced dosing frequency protocols in pharmaceutical markets.
The metered dose inhalers segment maintains 12.9% market share, serving respiratory disease management with precise pulmonary drug delivery. Transdermal & ocular patches hold 10.0% share, while drug-eluting stents & infusion pumps represent 20.3% market share, addressing cardiovascular and continuous infusion therapy applications.
Key market dynamics supporting application growth include:
- Oral delivery expansion driven by chronic disease management trends and patient compliance requirements, demanding convenient administration in pharmaceutical markets
- Injectable formulation growth trends require sophisticated, long-acting systems for biologics delivery and adherence differentiation
- Integration of smart delivery technologies enabling advanced patient monitoring capabilities and automated dosing systems
- Growing emphasis on personalized medicine driving demand for specialized, tailored drug delivery solutions without traditional administration limitations
What are the Drivers, Restraints, and Key Trends of the Controlled Release Drug Delivery Market?
The market is driven by three concrete demand factors tied to therapeutic performance outcomes. First, chronic disease prevalence and aging population demographics create increasing demand for specialized controlled release systems, with medication adherence representing 30-50% of therapeutic success in chronic conditions worldwide, requiring comprehensive delivery innovation. Second, pharmaceutical company initiatives promoting biologics development and precision medicine drive increased adoption of advanced drug delivery technologies, with many organizations implementing therapeutic optimization frameworks for enhanced clinical outcomes by 2030. Third, technological advancements in polymeric nanoparticles and microsphere formulations enable more efficient and targeted drug delivery solutions that improve therapeutic efficacy while reducing dosing frequency and adverse effects.
Market restraints include high regulatory approval requirements and clinical trial costs for controlled release formulations that can challenge market participants in developing compliant drug delivery capabilities, particularly in regions where pharmaceutical development resources remain limited and uncertain. Technical complexity of formulation optimization and bioequivalence demonstration requirements pose another significant challenge, as controlled release systems demand sophisticated manufacturing processes and extensive stability testing, potentially affecting development timelines and commercialization efficiency. Limited pediatric formulation availability and dose flexibility constraints across different patient populations create additional clinical challenges for pharmaceutical companies, demanding ongoing investment in age-appropriate delivery systems and patient-specific dosing solutions.
Key trends indicate accelerated adoption in Asia-Pacific markets, particularly India and China, where pharmaceutical manufacturing expansion and chronic disease burden drive comprehensive controlled release drug delivery adoption. Technology integration trends toward IoT-enabled smart injection devices with real-time monitoring, biodegradable polymer systems with enhanced biocompatibility, and AI-integrated bio-feedback loops enable efficient therapeutic approaches that optimize drug delivery and minimize medication errors. The growing emphasis on personalized medicine and companion diagnostics creates expanding opportunities for controlled release manufacturers across precision therapy and targeted treatment segments. However, the market thesis could face disruption if significant advances in gene therapy or major changes in pharmaceutical development paradigms reduce reliance on traditional controlled release delivery methods.
Analysis of the Controlled Release Drug Delivery Market by Key Country
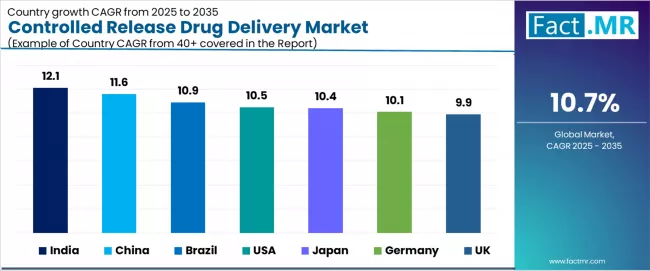
| Country | CAGR (2025-2035) |
|---|---|
| India | 12.1% |
| China | 11.6% |
| Brazil | 10.9% |
| USA | 10.5% |
| Japan | 10.4% |
| Germany | 10.1% |
| UK | 9.9% |
The global controlled release drug delivery market is expanding steadily, with India leading at a 12.1% CAGR through 2035, driven by rapid expansion of biopharmaceutical manufacturing and domestic pharmaceutical innovation. China follows at 11.6%, supported by diabetes and cardiovascular disease burden, government healthcare reforms, and pharmaceutical infrastructure development.
Brazil records 10.9%, reflecting chronic disease prevalence and expanding elderly population healthcare needs. USA grows at 10.5%, anchored by research and development leadership and chronic care focus. Japan advances at 10.4%, leveraging geriatric care demand and precision delivery technologies.
Germany posts 10.1%, emphasizing pharmaceutical innovation and aging population requirements, while UK grows steadily at 9.9%, focusing on personalized medicine integration and patient adherence programs.
India Leads Global Market Expansion
India demonstrates the strongest growth potential in the controlled release drug delivery market with a CAGR of 12.1% through 2035. The country's leadership position stems from rapid biopharmaceutical sector expansion, expanding domestic manufacturing capabilities, and comprehensive pharmaceutical regulations driving the adoption of advanced drug delivery solutions.
Growth is concentrated in major pharmaceutical manufacturing centers and innovation hubs, including Mumbai, Hyderabad, Bangalore, and Ahmedabad, where pharmaceutical companies and research organizations are implementing modern controlled release technologies for enhanced therapeutic delivery and patient compliance.
Distribution channels through pharmaceutical manufacturers and contract development organizations expand deployment across chronic disease management and specialty pharmaceutical initiatives. The country's Central Drugs Standard Control Organization provides regulatory support for controlled release technology adoption, including comprehensive drug approval frameworks.
Key market factors:
- Pharmaceutical manufacturing expansion concentrated in established hubs with comprehensive research and development programs
- Government support through healthcare initiatives and pharmaceutical manufacturing incentives
- Comprehensive drug development ecosystem, including established contract manufacturing organizations with proven formulation capabilities
- Technology integration featuring advanced polymer systems, biologics delivery platforms, and patient adherence technologies
China Emerges as High-Growth Market
In major pharmaceutical and healthcare centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of comprehensive controlled release drug delivery solutions is accelerating across chronic disease management and specialty pharmaceutical initiatives, driven by technology innovation and government healthcare reform programs. The market demonstrates strong growth momentum with a CAGR of 11.6% through 2035, linked to comprehensive pharmaceutical industry expansion and increasing focus on diabetes and cardiovascular disease management solutions.
Chinese pharmaceutical companies are implementing advanced controlled release systems and long-acting formulations to enhance therapeutic outcomes while meeting growing demand in expanding chronic care and specialty treatment sectors. The country's national healthcare reform initiatives create ongoing demand for innovative drug delivery technologies, while increasing emphasis on biologics development drives adoption of sophisticated delivery platforms.
Key development areas:
- Pharmaceutical companies and healthcare facilities leading controlled release adoption with comprehensive therapeutic management programs
- Drug development channels providing integrated solutions with high regulatory compliance rates
- Technology partnerships between international pharmaceutical companies and domestic manufacturers are expanding market reach
- Integration of precision medicine platforms and comprehensive patient monitoring systems
USA Shows Innovation Leadership
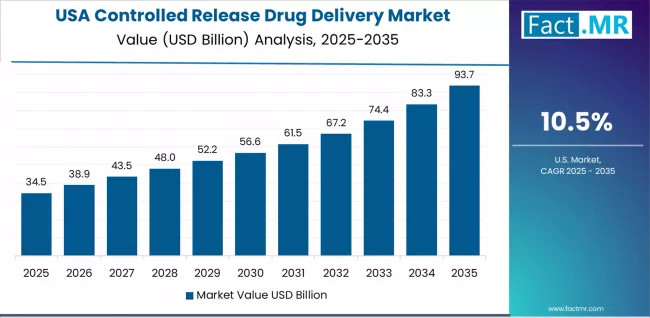
USA market expansion is driven by diverse pharmaceutical demand, including chronic disease management in major research centers and comprehensive drug development across multiple therapeutic areas. The country demonstrates strong growth potential with a CAGR of 10.5% through 2035, supported by federal research funding programs and pharmaceutical industry innovation initiatives.
American pharmaceutical companies face development opportunities related to biologics formulation and patient adherence optimization, requiring strategic investment in controlled release technologies through specialized research organizations. Growing chronic disease burden and personalized medicine requirements create compelling business cases for controlled release drug delivery adoption, particularly in oncology and neurological applications where advanced delivery systems have a direct impact on therapeutic success and patient quality of life.
Market characteristics:
- Pharmaceutical research and biotechnology segments showing robust growth with substantial annual increase in controlled release product development
- Regional innovation trends focused on advanced formulation activities in major pharmaceutical and biotechnology clusters
- Future projections indicate the need for advanced regulatory expertise infrastructure and specialized manufacturing capabilities
- Growing emphasis on patient adherence and therapeutic optimization competitiveness in pharmaceutical development
Germany Demonstrates Pharmaceutical Excellence
The Germany market leads in advanced controlled release innovation based on integration with precision medicine platforms and sophisticated formulation technologies for enhanced therapeutic performance. The country shows strong potential with a CAGR of 10.1% through 2035, driven by the development of pharmaceutical infrastructure and the expansion of advanced drug delivery systems in major pharmaceutical regions, including North Rhine-Westphalia, Baden-Württemberg, Bavaria, and Hesse.
German pharmaceutical companies are adopting intelligent controlled release systems for therapeutic improvement and regulatory compliance enhancement, particularly in regions with advanced quality requirements and pharmaceutical applications demanding comprehensive delivery optimization. Technology deployment channels through established pharmaceutical manufacturers and research institutes expand coverage across chronic disease management and specialty pharmaceutical projects.
Leading market segments:
- Pharmaceutical development projects in major research centers are implementing comprehensive controlled release technology solutions
- Technology partnerships with contract development organizations, achieving high therapeutic efficacy rates
- Strategic collaborations between pharmaceutical companies and drug delivery specialists are expanding market presence
- Focus on biologics delivery systems and specialized regulatory compliance requirements
UK Emphasizes Personalized Medicine Integration
In London, Manchester, Cambridge, and other major pharmaceutical centers, research organizations are implementing comprehensive controlled release solutions to advance therapeutic delivery capabilities and improve patient adherence, with documented case studies showing substantial improvement in treatment outcomes through advanced drug delivery integration. The market shows strong growth potential with a CAGR of 9.9% through 2035, linked to the ongoing development of pharmaceutical innovation, biotechnology advancement, and emerging precision medicine projects in major regions.
British pharmaceutical companies are adopting intelligent controlled release and patient monitoring platforms to enhance therapeutic reliability while maintaining standards demanded by the healthcare system and regulatory authorities. The country's established pharmaceutical infrastructure creates ongoing demand for delivery system innovation and therapeutic optimization solutions that integrate with existing treatment protocols.
Market development factors:
- Pharmaceutical companies and research organizations leading controlled release innovation initiatives across UK
- Healthcare programs providing government funding support for pharmaceutical research and therapeutic delivery development
- Strategic partnerships between British pharmaceutical firms and international drug delivery providers are expanding technical capabilities
- Emphasis on personalized medicine integration and patient adherence optimization across therapeutic applications
Japan Shows Technology Leadership
Japan's controlled release drug delivery market demonstrates sophisticated implementation focused on geriatric care optimization and precision therapeutic delivery, with documented integration of advanced delivery systems, achieving substantial improvement in patient adherence across pharmaceutical and healthcare facilities.
The country maintains strong growth momentum with a CAGR of 10.4% through 2035, driven by pharmaceutical companies' emphasis on quality excellence and continuous therapeutic improvement methodologies that align with Japanese healthcare standards applied to drug delivery operations.
Major pharmaceutical centers, including Kanto, Kansai, Chubu, and Kyushu, showcase advanced deployment of controlled release technologies where drug delivery systems integrate seamlessly with existing treatment protocols and comprehensive patient care programs.
Key market characteristics:
- Pharmaceutical companies and healthcare facilities are driving advanced controlled release requirements with emphasis on therapeutic precision and patient adherence
- Quality partnerships enabling high clinical compliance with comprehensive pharmaceutical development programs
- Technology collaboration between Japanese companies and international drug delivery providers is expanding market capabilities
- Emphasis on delivery system reliability requirements and continuous therapeutic optimization methodologies
Brazil Emphasizes Healthcare Development
In major metropolitan and healthcare centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of modern controlled release drug delivery solutions is expanding across pharmaceutical development and healthcare initiatives, driven by chronic disease prevalence and aging population growth. The market demonstrates strong growth potential with a CAGR of 10.9% through 2035, linked to comprehensive healthcare infrastructure development and increasing focus on therapeutic adherence solutions.
Brazilian pharmaceutical companies are implementing controlled release systems and modern formulation platforms to enhance therapeutic performance while meeting growing demand in expanding chronic disease management and specialty treatment sectors. The country's healthcare modernization initiatives create ongoing demand for advanced drug delivery technologies, while increasing emphasis on patient care quality drives adoption of controlled release formulations.
Key development areas:
- Pharmaceutical companies and healthcare facilities leading controlled release adoption with comprehensive therapeutic programs
- Drug delivery channels providing integrated solutions with expanding development capabilities
- Technology partnerships between international pharmaceutical manufacturers and local companies are expanding market reach
- Integration of patient monitoring platforms and comprehensive therapeutic management systems
Europe Market Split by Country
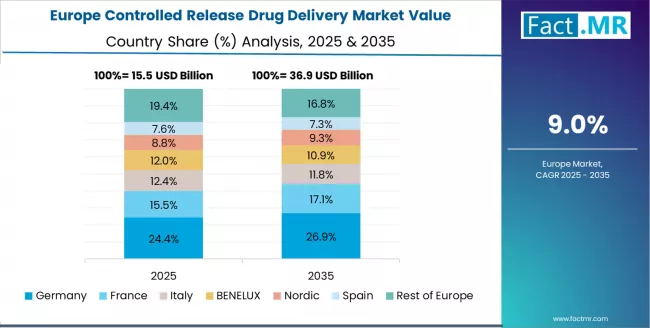
The controlled release drug delivery market in Europe is projected to grow from USD 17.8 billion in 2025 to USD 47.9 billion by 2035, registering a CAGR of 10.4% over the forecast period. Germany is expected to maintain its leadership position with a 27.0% market share in 2025, declining slightly to 26.9% by 2035, supported by its extensive pharmaceutical research infrastructure, advanced drug delivery innovation, and comprehensive biopharmaceutical development networks serving major European markets.
UK follows with a 19.1% share in 2025, projected to reach 19.0% by 2035, driven by comprehensive personalized medicine programs and pharmaceutical innovation initiatives implementing advanced controlled release systems. France holds a 18.4% share in 2025, expected to maintain 18.3% by 2035 through the ongoing development of pharmaceutical research and biotechnology networks.
Italy commands a 14.8% share, while Spain accounts for 11.2% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 9.5% to 9.7% by 2035, attributed to increasing controlled release adoption in Nordic countries and emerging Eastern European pharmaceutical facilities implementing advanced drug delivery programs.
Precision Medicine Dominates Pharmaceutical Demand in Japan
The Japanese controlled release drug delivery market demonstrates a mature and quality-focused landscape, characterized by sophisticated integration of high-precision delivery systems with existing pharmaceutical infrastructure across healthcare facilities, specialty pharmacies, and institutional providers. Japan's emphasis on quality excellence and therapeutic optimization drives demand for high reliability controlled release solutions that support comprehensive patient care initiatives and regulatory requirements in pharmaceutical operations.
The market benefits from strong partnerships between international pharmaceutical providers and domestic healthcare companies, including established pharmaceutical manufacturers and medical device enterprises, creating comprehensive therapeutic ecosystems that prioritize clinical quality and technical precision programs. Pharmaceutical centers in major urban regions showcase advanced controlled release implementations where drug delivery systems achieve therapeutic improvements through integrated patient monitoring programs.
Pharmaceutical Companies Lead Innovation Networks in South Korea
The South Korean controlled release drug delivery market is characterized by strong international pharmaceutical company presence, with organizations maintaining positions through comprehensive research and development integration and technical services capabilities for healthcare facilities and pharmaceutical applications.
The market is demonstrating a growing emphasis on localized formulation support and rapid commercialization capabilities, as South Korean pharmaceutical companies increasingly demand customized solutions that integrate with domestic healthcare infrastructure and advanced therapeutic protocols deployed across major medical centers and pharmaceutical facilities.
Local drug development organizations and regional pharmaceutical manufacturers are gaining market share through strategic partnerships with global providers, offering specialized services including technology transfer programs and regulatory support services for pharmaceutical professionals. The competitive landscape shows increasing collaboration between multinational pharmaceutical companies and Korean healthcare specialists, creating hybrid development models that combine international drug delivery expertise with local market knowledge and regulatory relationship capabilities.
Competitive Landscape of the Controlled Release Drug Delivery Market
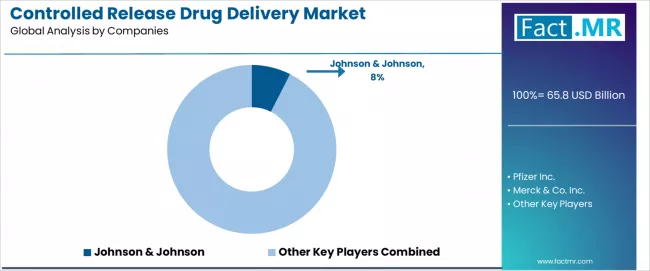
The controlled release drug delivery market features approximately 25-30 meaningful players with moderate concentration, where the top three companies control roughly 18-22% of global market share through established product portfolios and extensive research and development networks.
Competition centers on therapeutic efficacy, formulation innovation, and regulatory expertise rather than price competition alone. The industry demonstrates therapeutic area specialization patterns, with leading pharmaceutical companies maintaining strong positions in specific disease categories while expanding portfolios through strategic acquisitions and licensing agreements.
Market leaders include Johnson & Johnson, Pfizer Inc., and Merck & Co. Inc., which maintain competitive advantages through comprehensive controlled release drug portfolios, advanced formulation capabilities, and deep expertise in the pharmaceutical development sector, creating high prescriber loyalty among physicians and healthcare systems.
These companies leverage established clinical relationships and ongoing therapeutic area development partnerships to defend market positions while expanding into adjacent applications including biologics delivery and personalized medicine platforms. Johnson & Johnson demonstrates significant strength with approximately 7.5% market share in 2024, supported by expanding microsphere-based drug platforms in oncology applications.
Challengers encompass AbbVie Inc. and Novartis AG, which compete through specialized delivery technologies and strong therapeutic area presence in key pharmaceutical markets. Novartis AG has achieved notable success with the commercial launch of Leqvio (PCSK9 therapy) for cholesterol management, demonstrating long-acting injectable capabilities.
Controlled release specialists, including Bayer AG, Corium International Inc., and Alkermes plc, focus on specific delivery categories or therapeutic applications, offering differentiated capabilities in transdermal patch systems, psychiatric injectables, and specialized formulation technologies. Alkermes plc has demonstrated real-world adherence benefits with LYBALVI and ARISTADA in psychiatric care.
Recent competitive developments highlight strategic positioning shifts, with Pfizer Inc. advancing long-acting injectables for chronic pain management, AbbVie Inc. integrating lipid nanoparticle systems with oncology and neurological treatment lines, and AstraZeneca plc conducting research on microsphere and liposomal platforms.
Innovation-focused companies like Amneal Pharmaceuticals have secured FDA approvals for CREXONT and memantine/donepezil extended-release formulations, while Corium International Inc. has established collaborations with pharmaceutical firms for central nervous system therapies.
Market dynamics favor companies that combine advanced formulation technologies with comprehensive clinical development services that address the complete product lifecycle from research and formulation optimization through regulatory approval and commercial launch support.
Key Players in the Controlled Release Drug Delivery Market
- Johnson & Johnson
- Pfizer Inc.
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Bayer AG
- AstraZeneca plc
- Corium International Inc.
- Alkermes plc
- Amneal Pharmaceuticals
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 65.8 Billion |
| Technology | Targeted Delivery, Micro Encapsulation, Transdermal & Implants, Wurster & Coacervation Techniques, Others (Liposomes & Micro-electromechanical) |
| Application | Oral Controlled Drug Delivery, Injectable (Long-Acting Formulations), Metered Dose Inhalers, Transdermal & Ocular Patches, Drug-Eluting Stents & Infusion Pumps |
| Release Mechanism | Feedback-Regulated Systems, Activated Modulated Delivery Systems |
| Regions Covered | North America, Asia Pacific, Europe, Latin America, Middle East & Africa |
| Country Covered | USA, Germany, UK, China, India, Japan, Brazil, and 40+ countries |
| Key Companies Profiled | Johnson & Johnson, Pfizer Inc., Merck & Co. Inc., AbbVie Inc., Novartis AG, Bayer AG, AstraZeneca plc, Corium International Inc., Alkermes plc, Amneal Pharmaceuticals |
| Additional Attributes | Dollar sales by technology and application categories, regional adoption trends across North America, Asia Pacific, and Europe, competitive landscape with pharmaceutical manufacturers and drug delivery companies, formulation specifications and therapeutic requirements, integration with biologics development initiatives and personalized medicine platforms, innovations in controlled release technology and polymer systems, and development of specialized applications with patient adherence and therapeutic optimization capabilities. |
Controlled Release Drug Delivery Market by Segments
-
Technology :
- Targeted Delivery
- Micro Encapsulation
- Transdermal & Implants
- Wurster & Coacervation Techniques
- Others (Liposomes & Micro-electromechanical)
-
Application :
- Oral Controlled Drug Delivery
- Injectable (Long-Acting Formulations)
- Metered Dose Inhalers
- Transdermal & Ocular Patches
- Drug-Eluting Stents & Infusion Pumps
-
Release Mechanism :
- Feedback-Regulated Systems
- Activated Modulated Delivery Systems
-
Region :
- North America
- USA
- Canada
- Mexico
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Technology
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Technology, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Technology, 2025 to 2035
- Targeted Delivery
- Micro Encapsulation
- Transdermal & Implants
- Wurster & Coacervation Techniques
- Others (Liposomes & Micro-electromechanical)
- Y to o to Y Growth Trend Analysis By Technology, 2020 to 2024
- Absolute $ Opportunity Analysis By Technology, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Oral Controlled Drug Delivery
- Injectable (Long-Acting Formulations)
- Metered Dose Inhalers
- Transdermal & Ocular Patches
- Drug-Eluting Stents & Infusion Pumps
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Technology
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Technology
- By Application
- Competition Analysis
- Competition Deep Dive
- Johnson & Johnson
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Pfizer Inc.
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Bayer AG
- AstraZeneca plc
- Corium International Inc.
- Alkermes plc
- Amneal Pharmaceuticals
- Johnson & Johnson
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Technology
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Technology
- Figure 23: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Application
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Technology
- Figure 30: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by Application
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Technology
- Figure 37: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by Application
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Technology
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Application
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Technology
- Figure 51: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by Application
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Technology
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Technology
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the controlled release drug delivery market in 2025?
The global controlled release drug delivery market is estimated to be valued at USD 65.8 billion in 2025.
What will be the size of controlled release drug delivery market in 2035?
The market size for the controlled release drug delivery market is projected to reach USD 181.2 billion by 2035.
How much will be the controlled release drug delivery market growth between 2025 and 2035?
The controlled release drug delivery market is expected to grow at a 10.7% CAGR between 2025 and 2035.
What are the key product types in the controlled release drug delivery market?
The key product types in controlled release drug delivery market are targeted delivery, micro encapsulation, transdermal & implants, wurster & coacervation techniques and others (liposomes & micro-electromechanical).
Which application segment to contribute significant share in the controlled release drug delivery market in 2025?
In terms of application, oral controlled drug delivery segment to command 35.3% share in the controlled release drug delivery market in 2025.