Molecular Diagnostics For STD Market
Molecular Diagnostics For STD Market Size and Share Forecast Outlook 2025 to 2035
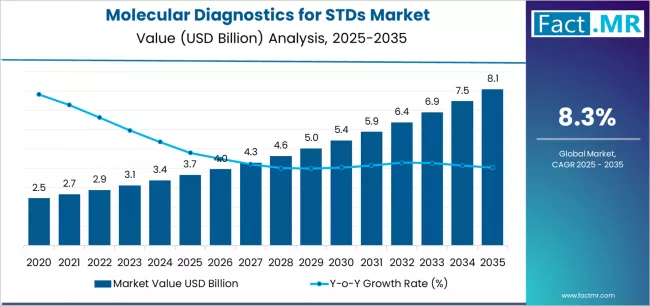
Molecular diagnostics for std market is projected to grow from USD 3.7 billion in 2025 to USD 8.1 billion by 2035, at a CAGR of 8.3%. Consumables (Reagents and Kits) will dominate with a 53.7% market share, while hiv testing will lead the application segment with a 38.4% share.
Molecular Diagnostics for STDs Market forecast and Outlook 2025 to 2035
The global molecular diagnostics for STDs market is projected to reach USD 8.08 billion by 2035, recording an absolute increase of USD 4.43 billion over the forecast period. The market is valued at USD 3.65 billion in 2025 and is set to rise at a CAGR of 8.3% during the assessment period.
The market is expected to grow by approximately 2.2 times during the same period, supported by increasing prevalence of sexually transmitted infections worldwide, driving demand for accurate diagnostic solutions and increasing investments in point-of-care testing technologies with rapid turnaround times across clinical, public health, and commercial laboratory applications globally.
Quick Stats for Molecular Diagnostics for STDs Market
- Molecular Diagnostics for STDs Market Value (2025): USD 3.65 billion
- Molecular Diagnostics for STDs Market forecast Value (2035): USD 8.08 billion
- Molecular Diagnostics for STDs Market forecast CAGR: 8.3%
- Leading Product Type in Molecular Diagnostics for STDs Market: Consumables (Reagents and kits) (53.7%)
- Key Growth Regions in Molecular Diagnostics for STDs Market: Asia Pacific, North America, and Europe
- Top Players in Molecular Diagnostics for STDs Market: F. Hoffmann-La Roche, BD, Hologic, Abbott, Cepheid (Danaher), Qiagen, OraSure Technologies, bioMérieux, Bio-Rad Laboratories, Thermo Fisher Scientific
Healthcare providers face mounting pressure to improve screening rates and enable early detection while addressing asymptomatic infections and antimicrobial resistance concerns, with modern molecular diagnostic platforms providing documented clinical benefits including high sensitivity and specificity, multiplex testing capabilities, and same-day results compared to conventional culture-based methods alone.
Rising awareness about STDs prevention and expanding access to diagnostic testing through public health initiatives create substantial opportunities for manufacturers and laboratory service providers. However, high test costs and reimbursement limitations across markets may pose obstacles to universal screening implementation.
The consumables segment dominates market activity, driven by recurring reagent and kit consumption across routine STDs screening programs and continuous testing demand supporting disease surveillance worldwide. Clinical laboratories increasingly recognize the operational advantages of molecular testing platforms, with typical consumable offerings providing reliable pathogen detection and workflow integration at established per-test economics through comprehensive diagnostic supply networks.
The instruments & services segment demonstrates substantial presence, supported by ongoing installation of automated molecular platforms and comprehensive service contracts. HIV testing emerges as the dominant application segment, reflecting the substantial global disease burden and established screening protocols across diverse healthcare settings. HPV testing represents the second-largest application, driven by cervical cancer prevention programs and expanding molecular-based screening guidelines.
Regional dynamics show North America maintaining market leadership, supported by comprehensive STDs screening recommendations and established reimbursement frameworks across public and private healthcare systems. Asia Pacific demonstrates the fastest growth trajectory driven by large population bases, increasing disease awareness, and expanding laboratory infrastructure, while Europe emphasizes national screening programs and comprehensive sexual health services.
China leads country-level growth through massive population screening initiatives and expanding healthcare access, followed by India supported by rising disease burden awareness and laboratory network development. The competitive landscape features moderate concentration with F. Hoffmann-La Roche maintaining market leadership position at a 12.4% market share through comprehensive molecular diagnostics portfolios, while established players including BD, Hologic, and Abbott compete through innovative testing platforms and extensive clinical laboratory partnerships across diverse STDs testing applications.
Molecular Diagnostics for STDs Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 3.65 billion |
| Market forecast Value (2035) | USD 8.08 billion |
| forecast CAGR (2025-2035) | 8.3% |
Why is the Molecular Diagnostics for STDs Market Growing?
The molecular diagnostics for STDs market expand by enabling healthcare providers to achieve accurate pathogen identification and enable timely treatment initiation while addressing asymptomatic infections and preventing disease transmission without exclusive reliance on culture-based methods.
Healthcare systems face mounting pressure to improve STD screening rates and reduce infection burden while managing antimicrobial resistance and contact tracing requirements, with modern molecular diagnostic platforms typically providing targeted capabilities including nucleic acid amplification, multiplex pathogen detection, and rapid result delivery compared to traditional microbiological techniques alone, making molecular testing essential for comprehensive sexual health management protocols.
Rising STDs prevalence rates and increasing chlamydia, gonorrhea, and syphilis incidence create expanding testing demand, with public health agencies requiring comprehensive screening programs to control disease transmission and identify asymptomatic carriers through routine molecular testing.
Growing point-of-care testing adoption and decentralized diagnostic capabilities drive market expansion, with healthcare providers demonstrating significant interest in rapid molecular platforms enabling same-visit diagnosis and treatment improving patient outcomes and reducing loss to follow-up. Increasing cervical cancer screening programs and expanding HPV testing guidelines enable market growth for molecular diagnostics supporting early detection and risk stratification across diverse female populations.
Segmental Analysis
The market is segmented by product, application, and technology. By product, the market is divided into consumables (reagents and kits), instruments & services, and software. Based on application, the market is categorized into HIV testing, HPV testing, CT/NG testing, syphilis testing, gonorrhea testing, HSV testing, trichomonas, ureaplasma + mycoplasma, and others. By technology, the market includes laboratory testing (commercial/private labs and public health labs) and point-of-care testing.
Which Category Leads by Product in the Molecular Diagnostics for STDs Market?
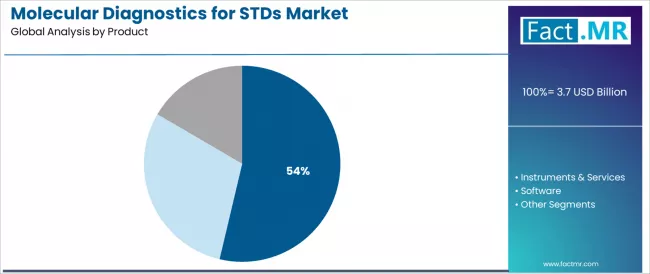
The consumables segment represents the dominant force in the molecular diagnostics for STDs market, capturing 53.7% of the total market share in 2025. This established product category encompasses reagents, kits, and assay components featuring essential nucleic acid amplification and detection capabilities, including PCR reagents, probe sets, sample preparation kits, and quality control materials that enable routine STDs testing across clinical laboratories, public health facilities, and point-of-care settings worldwide.
The consumables segment's market leadership stems from its recurring revenue nature and direct correlation with testing volumes, with products capable of providing reliable pathogen detection, standardized protocols, and regulatory compliance while maintaining cost-effectiveness standards across diverse laboratory workflows and testing platforms.
The instruments & services segment maintains substantial market presence at approximately 32.5%, serving capital equipment requirements for automated molecular platforms, real-time PCR systems, and sample-to-answer devices requiring installation, training, and ongoing technical support. These offerings provide critical infrastructure enabling high-throughput testing operations and comprehensive service agreements supporting equipment uptime and performance validation.
The software segment accounts for remaining market share, encompassing laboratory information management systems, data analysis tools, and connectivity solutions supporting result reporting and epidemiological surveillance.
Key commercial advantages driving the consumables segment include recurring revenue generation through continuous test kit consumption supporting stable business models and predictable demand patterns, direct scalability with testing volume growth enabling market expansion aligned with increasing STDs screening programs and disease surveillance initiatives, standardized performance characteristics supporting regulatory approval and quality assurance requirements across diverse laboratory settings, and broad platform compatibility enabling reagent utilization across multiple instrument systems and testing configurations supporting flexible laboratory operations.
By Application, Which STD Type Profiling uses Molecular Diagnostics Most Frequently?
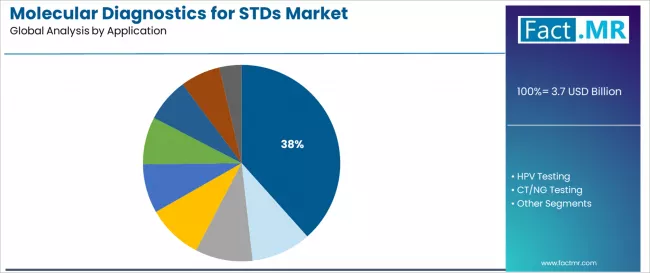
HIV testing represents the dominant application segment in the molecular diagnostics for STDs market with a 38.4% market share in 2025, reflecting the substantial global disease burden, established screening guidelines, and critical importance of early detection for treatment initiation and transmission prevention.
The HIV testing segment demonstrates robust demand driven by universal screening recommendations, viral load monitoring requirements for treatment management, and infant diagnosis applications requiring molecular methods for definitive early detection.
HPV testing emerges as the second-largest application category with a 21.1% market share in 2025, demonstrating significant growth potential driven by cervical cancer screening programs, expanding vaccination monitoring, and evolving clinical guidelines recommending molecular HPV testing as primary screening modality. Women's health applications require high-risk HPV genotyping supporting risk stratification and clinical decision-making.
CT/NG testing accounts for substantial market share, serving chlamydia and gonorrhea screening applications representing the most common bacterial STDss requiring routine surveillance. Other applications including syphilis testing, HSV testing, trichomonas, and ureaplasma + mycoplasma detection serve specialized diagnostic requirements across comprehensive STDs screening panels.
Key application dynamics include HIV testing dominance driven by established diagnostic algorithms and treatment monitoring requirements supporting lifelong patient management, HPV testing growth reflecting cervical cancer prevention program expansion and primary screening guideline adoption globally, CT/NG testing prevalence supporting routine screening recommendations in sexually active populations and high-risk groups, and multiplex testing adoption enabling comprehensive STDs panels detecting multiple pathogens simultaneously improving diagnostic efficiency and patient convenience.
What are the Drivers, Restraints, and Key Trends of the Molecular Diagnostics for STDs Market?
The market is driven by three concrete demand factors tied to public health outcomes. First, rising STDs prevalence rates and increasing incidence of chlamydia, gonorrhea, and syphilis create expanding testing demand, with CDC reporting over 2.5 million combined cases annually in USA alone requiring comprehensive screening programs and routine molecular testing for disease control, demanding widespread diagnostic availability. Second, growing point-of-care testing adoption and decentralized diagnostic implementation drive market expansion, with healthcare providers demonstrating significant investment in rapid molecular platforms enabling same-visit diagnosis and treatment improving cure rates and reducing transmission through immediate intervention by 2030. Third, increasing cervical cancer screening programs and expanding HPV testing recommendations enable market growth for molecular diagnostics supporting primary screening strategies and risk-based management across diverse female populations aligned with WHO elimination goals.
Market restraints include high test costs and limited reimbursement coverage that can challenge routine screening implementation, particularly in resource-limited settings where per-test economics exceed available healthcare budgets and screening frequency recommendations strain system capacity. Stigma and privacy concerns surrounding STDs testing pose another significant obstacle, as patients may avoid screening due to confidentiality fears and social implications, potentially affecting testing volumes and disease control program effectiveness. Technical complexity and laboratory infrastructure requirements create additional barriers for market penetration in developing regions, demanding trained personnel, quality assurance programs, and equipment maintenance capabilities not universally available across healthcare systems.
Key trends indicate accelerated point-of-care testing adoption in developed markets, particularly USA and Europe, where healthcare providers demonstrate willingness to invest in rapid molecular platforms supporting immediate clinical decision-making and treatment initiation during single patient visits. Multiplex testing panel expansion trends toward comprehensive STDs screening detecting multiple pathogens simultaneously optimize workflow efficiency and diagnostic completeness while addressing co-infection patterns. However, the market thesis could face disruption if significant advances in rapid antigen testing provide acceptable performance at substantially lower costs or major shifts in disease epidemiology through successful vaccination programs reduce testing demand for specific pathogens.
Analysis of the Molecular Diagnostics for STDs Market by Key Countries
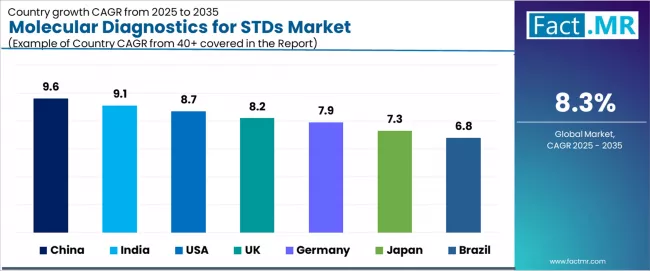
| Country | CAGR (2025 to 2035) |
|---|---|
| China | 9.6% |
| India | 9.1% |
| USA | 8.7% |
| UK | 8.2% |
| Germany | 7.9% |
| Japan | 7.3% |
| Brazil | 6.8% |
The global molecular diagnostics for STDs market is expanding rapidly, with China leading at a 9.6% CAGR through 2035, driven by massive population screening initiatives, expanding laboratory network infrastructure, and government-supported disease control programs. India follows at 9.1%, supported by rising disease burden awareness, expanding diagnostic laboratory capacity, and increasing healthcare access in urban centers. USA records 8.7%, reflecting comprehensive screening guidelines, established reimbursement frameworks, and point-of-care testing adoption.
UK advances at 8.2%, leveraging national sexual health services and comprehensive screening programs through NHS. Germany posts 7.9%, focusing on established laboratory infrastructure and comprehensive health insurance coverage, while Japan grows at 7.3%, emphasizing quality diagnostic standards and aging population healthcare needs. Brazil demonstrates 6.8% growth, anchored by public health system expansion and STDs control program development.
What Makes China the Fastest Growing Market Globally?
China demonstrates the strongest growth potential in the molecular diagnostics for STDs market with a CAGR of 9.6% through 2035. The country's leadership position stems from massive population base, expanding healthcare infrastructure, and government commitment to disease control programs requiring comprehensive STDs screening capabilities.
Growth is concentrated in major urban centers and provincial laboratories, including CDC network facilities where molecular diagnostic platforms are increasingly deployed for surveillance and clinical testing supporting public health objectives. Distribution channels through hospital laboratories, independent clinical laboratories, and public health institutions expand testing accessibility across diverse populations.
The country's emphasis on infectious disease control and expanding sexual health awareness provides strong momentum for molecular testing adoption, including comprehensive implementation across both urban tertiary hospitals and expanding county-level healthcare facilities.
Key market factors include population-scale screening initiatives through National Health Commission programs supporting STDs control and prevention strategies requiring molecular diagnostic infrastructure, laboratory network expansion including independent clinical laboratories and hospital-based facilities increasing testing capacity and geographic coverage, HIV/AIDS control programs requiring molecular diagnostics for viral load monitoring and infant diagnosis supporting treatment program effectiveness, and domestic manufacturer development featuring Chinese diagnostic companies developing molecular platforms and reagents supporting cost-effective testing scale-up.
How is India Emerging as a High-Growth Market?
In major urban centers including Delhi, Mumbai, Bangalore, and Chennai, the adoption of molecular STDs diagnostics is accelerating across private laboratories and tertiary care hospitals, driven by rising disease awareness and expanding middle-class healthcare access. The market demonstrates strong growth momentum with a CAGR of 9.1% through 2035, linked to increasing STDs burden recognition and healthcare infrastructure modernization.
Indian laboratories are implementing molecular testing platforms to support clinical diagnosis and enable evidence-based treatment while meeting growing demand for accurate pathogen detection. The country's large population and expanding diagnostic laboratory sector create substantial market opportunities, while government health programs and targeted interventions support testing scale-up for priority diseases.
Key development areas include urban laboratory expansion through private chains including Thyrocare, Dr. Lal PathLabs, and regional players establishing molecular testing capabilities, disease burden recognition driving awareness about STDs prevalence including HIV, HPV, and bacterial infections requiring diagnostic infrastructure, government programs through National AIDS Control Organization and reproductive health initiatives supporting testing access and treatment linkage, and healthcare infrastructure development including expansion of medical colleges and district hospitals implementing modern diagnostic capabilities.
What Drives USA Market Leadership?
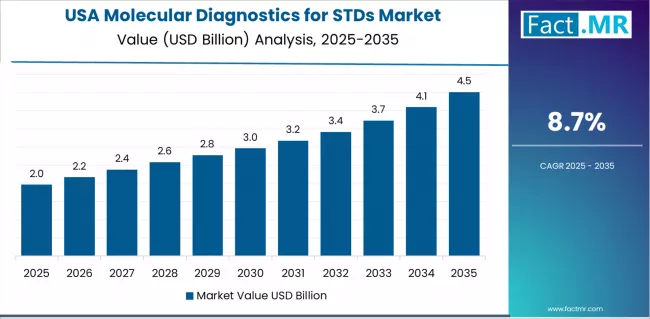
USA market expansion is driven by comprehensive screening guidelines, including CDC recommendations for routine chlamydia and gonorrhea screening in sexually active women and targeted testing in high-risk populations. The country demonstrates strong growth potential with a CAGR of 8.7% through 2035, supported by established molecular diagnostic infrastructure, extensive clinical laboratory networks, and emerging point-of-care testing adoption. American healthcare providers face increasing STDs rates requiring enhanced screening efforts and comprehensive testing strategies. The country's advanced diagnostic industry and insurance coverage frameworks support widespread molecular testing access across diverse clinical settings.
Market characteristics include comprehensive screening recommendations through CDC guidelines supporting routine molecular testing in primary care and sexual health clinics, point-of-care adoption through FDA-approved rapid molecular platforms including Cepheid GeneXpert and other systems enabling same-visit diagnosis and treatment, commercial laboratory dominance through national chains including Quest Diagnostics and LabCorp providing comprehensive STDs testing panels, and public health infrastructure including state and local health departments operating laboratories supporting disease surveillance and partner notification programs.
How Does UK Demonstrate National Health Service Integration?
The UK market leads in integrated sexual health service delivery based on comprehensive NHS-funded screening programs and specialized genitourinary medicine clinics. The country shows strong potential with a CAGR of 8.2% through 2035, driven by national sexual health strategy and established screening pathways. The sexual health services implement molecular diagnostics through integrated care models combining testing, treatment, and partner notification services. The country's universal healthcare system and dedicated sexual health clinics create comprehensive testing infrastructure supporting high screening rates and disease control.
Leading market segments include NHS sexual health clinics providing free STDs testing and treatment services with molecular diagnostic capabilities, national screening programs including cervical screening with HPV testing and opportunistic chlamydia screening targeting young adults, laboratory consolidation through regional reference laboratories implementing high-throughput molecular platforms supporting national testing volumes, and public health surveillance through Public Health England monitoring STDs epidemiology and resistance patterns supporting evidence-based policy development.
What Positions Germany for Laboratory Excellence?
Germany's molecular diagnostics for STDs market demonstrates sophisticated laboratory infrastructure focused on quality standards and comprehensive testing protocols. The country maintains steady growth momentum with a CAGR of 7.9% through 2035, driven by established healthcare system and universal insurance coverage supporting diagnostic access. German laboratories implement molecular testing through certified facilities adhering to stringent quality requirements and medical guidelines. The country's comprehensive health insurance system and preventive care emphasis create stable demand for STDs screening services.
Key market characteristics include universal health insurance coverage through statutory and private systems supporting STDs testing reimbursement and patient access, quality-certified laboratories implementing molecular diagnostics through facilities meeting German medical laboratory standards and external quality assessment, preventive care programs including sexual health counseling and screening recommendations supporting early detection, and research leadership through academic medical centers and diagnostic companies advancing molecular testing technologies and clinical applications.
What Characterizes Japan's Market Development?
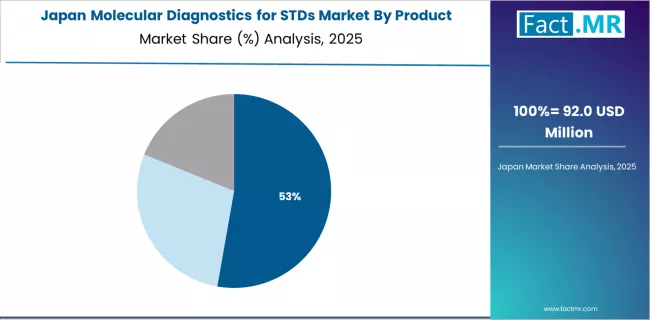
Japan demonstrates mature market characteristics with emphasis on quality diagnostics and established healthcare delivery systems. The country shows meaningful growth potential with a CAGR of 7.3% through 2035, driven by aging population healthcare needs and comprehensive screening programs. Japanese laboratories implement molecular STDs diagnostics through sophisticated platforms emphasizing accuracy and reliability. The country's universal health insurance and advanced medical technology adoption support consistent testing demand across clinical settings.
Key development factors include universal health coverage through national health insurance providing STDs testing access across population, quality-focused diagnostics emphasizing high-performance molecular platforms and rigorous quality control standards, cervical cancer screening programs incorporating HPV testing supporting primary prevention strategies, and aging population considerations including age-specific screening recommendations and healthcare utilization patterns.
What Drives Brazil's Public Health Expansion?
Brazil demonstrates meaningful growth potential with a CAGR of 6.8% through 2035, driven by SUS public health system expansion and STDs control program development. Brazilian health authorities are implementing molecular diagnostics to support HIV/AIDS programs and expand STDs screening capabilities across diverse populations. The country's large population and universal health system create opportunities for testing scale-up, while private sector laboratory chains serve urban populations with comprehensive diagnostic services.
Key development areas include SUS public health system providing free STDs testing and treatment services supporting disease control objectives, HIV/AIDS programs through Ministry of Health implementing molecular diagnostics for viral load monitoring and infant diagnosis, private laboratory sector through chains including Dasa and Fleury providing comprehensive STDs testing panels in urban markets, and disease burden addressing high STDs prevalence requiring enhanced screening and prevention strategies.
Europe Market Split by Country
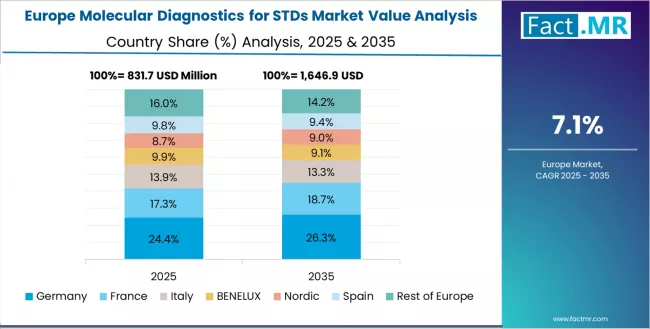
The molecular diagnostics for STDs market in Europe is projected to grow from USD 0.95 billion in 2025 to USD 2.10 billion by 2035, registering a CAGR of 8.3% over the forecast period. UK is expected to maintain its leadership position with a 28.8% market share in 2025, adjusting to 28.3% by 2035, supported by its comprehensive NHS sexual health services, national screening programs, and established GUM clinic networks serving major populations.
Germany follows with a 26.5% share in 2025, projected to reach 26.8% by 2035, driven by universal health insurance coverage, certified laboratory infrastructure, and comprehensive preventive care programs implementing molecular STDs testing protocols. France holds a 21.2% share in 2025, expected to maintain 21.5% by 2035 through public health initiatives and sexual health service expansion.
Italy commands a 13.5% share, while Spain accounts for 10.0% in 2025. The rest of Europe is anticipated to maintain stable presence, with collective share remaining steady by 2035, attributed to consistent molecular diagnostic adoption across Nordic countries and smaller European markets implementing sexual health programs.
Competitive Landscape of the Molecular Diagnostics for STDs Market
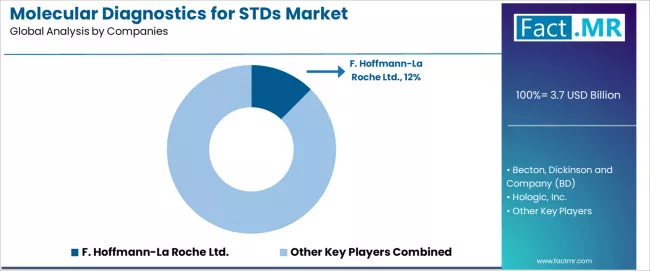
The molecular diagnostics for STDs market features approximately 12-15 meaningful players with moderate concentration, where F. Hoffmann-La Roche Ltd. maintains a 12.4% of global market share through comprehensive molecular diagnostics portfolios, established cobas platform presence, and extensive clinical laboratory partnerships. Competition centers on assay performance, platform automation, and menu breadth rather than price alone, with manufacturers differentiating through multiplex capabilities, turnaround times, and workflow integration.
Market leaders include F. Hoffmann-La Roche, BD, and Hologic, which maintain competitive advantages through FDA-cleared STDs testing panels, automated sample-to-answer platforms, and deep relationships with clinical laboratories, hospital systems, and public health institutions. These companies leverage regulatory approval portfolios and ongoing assay development to defend market positions while expanding offerings including point-of-care systems and comprehensive multiplex panels addressing evolving clinical needs.
Established diagnostic companies encompass Abbott, Cepheid (Danaher), and Qiagen, which compete through diverse molecular platforms, rapid testing capabilities, and global commercial infrastructure. Specialty players including OraSure Technologies focus on point-of-care and alternative specimen testing, while bioMérieux, Bio-Rad Laboratories, and Thermo Fisher Scientific offer comprehensive molecular diagnostic solutions across multiple infectious disease applications including STDs.
Emerging point-of-care developers and regional diagnostic companies create competitive pressure through innovative testing formats and cost-effective solutions, particularly in high-growth markets where decentralized testing models provide advantages in patient access and same-visit treatment initiation. Market dynamics favor companies combining analytical performance with workflow efficiency and comprehensive clinical evidence supporting guideline-recommended screening protocols.
Strategic emphasis on multiplex panel development, point-of-care platform innovation, and laboratory information system integration enables differentiation in increasingly competitive molecular diagnostics markets across clinical, public health, and commercial laboratory segments.
Key Players in the Molecular Diagnostics for STDs Market
- F. Hoffmann-La Roche Ltd.
- Becton, Dickinson and Company (BD)
- Hologic, Inc.
- Abbott Laboratories
- Cepheid (a Danaher company)
- QIAGEN N.V.
- OraSure Technologies, Inc.
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 3.65 Billion |
| Product | Consumables (Reagents and kits), Instruments & Services, Software |
| Application | HIV Testing, HPV Testing, CT/NG Testing, Syphilis Testing, Gonorrhea Testing, HSV Testing, Trichomonas, Ureaplasma + Mycoplasma, Others |
| Technology | Laboratory Testing (Commercial/Private Labs, Public Health Labs), Point-of-Care Testing |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | China, India, USA, UK, Germany, Japan, Brazil, and 40+ countries |
| Key Companies Profiled | F. Hoffmann-La Roche, BD, Hologic, Abbott, Cepheid (Danaher), Qiagen, OraSure Technologies, bioMérieux, Bio-Rad Laboratories, Thermo Fisher Scientific |
| Additional Attributes | Dollar sales by product and application categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with diagnostic manufacturers and laboratory service providers, assay specifications and clinical performance requirements, integration with laboratory information systems and screening programs, innovations in point-of-care platforms and multiplex testing, and development of specialized applications with clinical guideline alignment and public health surveillance capabilities. |
Molecular Diagnostics for STDs Market by Segments
-
Product :
- Consumables (Reagents and Kits)
- Instruments & Services
- Software
-
Application :
- HIV Testing
- HPV Testing
- CT/NG Testing
- Syphilis Testing
- Gonorrhea Testing
- HSV Testing
- Trichomonas
- Ureaplasma + Mycoplasma
- Others
-
Technology :
- Laboratory Testing
- Commercial/Private Labs
- Public Health Labs
- Point-of-Care Testing
- Laboratory Testing
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Consumables (Reagents and Kits)
- Instruments & Services
- Software
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- HIV Testing
- HPV Testing
- CT/NG Testing
- Syphilis Testing
- Gonorrhea Testing
- HSV Testing
- Trichomonas
- Ureaplasma + Mycoplasma
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Product
- By Application
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Application
- Competition Analysis
- Competition Deep Dive
- F. Hoffmann-La Roche Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Becton, Dickinson and Company (BD)
- Hologic, Inc.
- Abbott Laboratories
- Cepheid (a Danaher company)
- QIAGEN N.V.
- OraSure Technologies, Inc.
- bioMérieux S.A.
- Bio-Rad Laboratories, Inc.
- Thermo Fisher Scientific Inc.
- F. Hoffmann-La Roche Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Product
- Figure 6: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Application
- Figure 9: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by Region
- Figure 12: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 14: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 15: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 16: USA Market Attractiveness Analysis by Product
- Figure 17: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Application
- Figure 20: USA Market - Tier Structure Analysis
- Figure 21: USA Market - Company Share Analysis
- FAQs -
How big is the molecular diagnostics for std market in 2025?
The global molecular diagnostics for std market is estimated to be valued at USD 3.7 billion in 2025.
What will be the size of molecular diagnostics for std market in 2035?
The market size for the molecular diagnostics for std market is projected to reach USD 8.1 billion by 2035.
How much will be the molecular diagnostics for std market growth between 2025 and 2035?
The molecular diagnostics for std market is expected to grow at a 8.3% CAGR between 2025 and 2035.
What are the key product types in the molecular diagnostics for std market?
The key product types in molecular diagnostics for std market are consumables (reagents and kits), instruments & services and software.
Which application segment to contribute significant share in the molecular diagnostics for std market in 2025?
In terms of application, hiv testing segment to command 38.4% share in the molecular diagnostics for std market in 2025.