Neonatal Toxicology Market
Neonatal Toxicology Market Size and Share Forecast Outlook 2025 to 2035
Neonatal toxicology market is projected to grow from USD 310.2 million in 2025 to USD 879.6 million by 2035, at a CAGR of 11.0%. Meconium will dominate with a 39.2% market share, while mass spectroscopy will lead the technology segment with a 67.1% share.
Neonatal Toxicology Market Forecast and Outlook 2025 to 2035
The global neonatal toxicology market is projected to reach USD 879.6 million by 2035, recording an absolute increase of USD 569.4 million over the forecast period. The market is valued at USD 310.2 million in 2025 and is set to rise at a CAGR of 11.0% during the assessment period.
Quick Stats for Neonatal Toxicology Market
- Neonatal Toxicology Market Value (2025): USD 310.2 million
- Neonatal Toxicology Market Forecast Value (2035): USD 879.6 million
- Neonatal Toxicology Market Forecast CAGR: 11.0%
- Leading Specimen Type in Neonatal Toxicology Market: Meconium (39.2%)
- Key Growth Regions in Neonatal Toxicology Market: Asia Pacific, North America, and Europe
- Top Players in Neonatal Toxicology Market: Quest Diagnostics, LabCorp, Quidel Corporation, Bio-Rad Laboratories, Clinical Reference Laboratory (CRL), Omega Laboratories, Cordant Health Solutions, Agilent Technologies, USDTL, Thermo Fisher Scientific
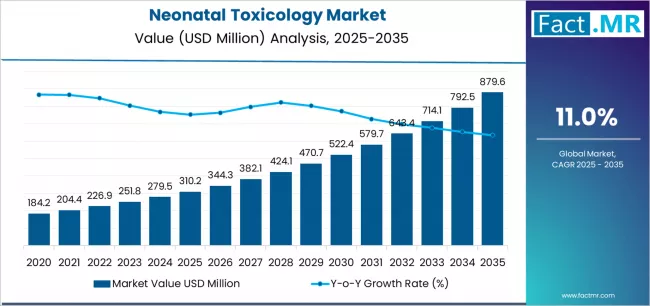
The overall market size is expected to grow by approximately 2.8 times during the same period, supported by increasing maternal substance abuse prevalence and expanding mandatory newborn screening programs worldwide, driving demand for comprehensive drug testing solutions and increasing investments in advanced mass spectrometry technologies with enhanced detection sensitivity across meconium and umbilical cord specimen analysis globally.
Healthcare providers and neonatal care specialists face mounting pressure to identify in-utero drug exposure early and implement appropriate intervention protocols while managing complex maternal histories and legal reporting requirements, with modern neonatal toxicology testing providing documented clinical advantages including precise drug identification, extended detection windows, and comprehensive substance panels compared to traditional maternal self-reporting alone.
Rising opioid epidemic impacts and expanding NICU infrastructure development across emerging economies create substantial opportunities for diagnostic laboratories and technology providers. However, ethical concerns regarding maternal privacy and high testing costs may pose obstacles to universal screening implementation.
The meconium segment dominates market activity, driven by superior detection window characteristics and comprehensive drug exposure history enabling effective clinical assessment worldwide. Neonatal healthcare providers increasingly utilize meconium-based testing, with typical specimen offerings providing extended detection capabilities covering entire gestational periods through established collection protocols.
The umbilical cord segment demonstrates robust growth potential, supported by convenient collection procedures and reliable drug detection capabilities enabling practical implementation in modern birthing facilities.
Mass spectroscopy emerges as the dominant technology segment, reflecting superior analytical sensitivity and specificity essential for confirmatory drug identification. Clinical laboratories maintain market leadership among end-use segments, while hospital NICUs gain momentum through point-of-care testing adoption.
Regional dynamics show significant growth potential across Asia Pacific, supported by rising maternal drug exposure awareness and expanding neonatal care infrastructure across China, India, and Southeast Asian markets.
North America demonstrates strong market presence driven by established screening protocols and high prenatal substance abuse prevalence, while Europe emphasizes early intervention programs and preventive healthcare frameworks. India leads country-level growth through rapid NICU expansion and rising maternal drug exposure recognition, followed by China supported by healthcare reforms and increased screening adoption.
The competitive landscape features moderate concentration with Quest Diagnostics maintaining market leadership position, while established players including LabCorp, Quidel Corporation, and Bio-Rad Laboratories compete through comprehensive testing portfolios and advanced analytical capabilities across diverse neonatal toxicology applications.
Neonatal Toxicology Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the neonatal toxicology market is projected to expand from USD 310.2 million to USD 469.3 million, resulting in a value increase of USD 159.1 million, which represents 27.9% of the total forecast growth for the period. This phase of development will be shaped by rising demand for comprehensive multi-drug screening panels addressing opioid and cannabinoid exposure, product innovation in rapid point-of-care immunoassays with improved sensitivity specifications, as well as expanding integration with electronic health record systems and automated laboratory information management platforms. Companies are establishing competitive positions through investment in LC-MS/MS technology upgrades, advanced specimen processing capabilities, and strategic market expansion across hospital laboratories, independent reference labs, and NICU facilities.
From 2029 to 2035, the market is forecast to grow from USD 469.3 million to USD 879.6 million, adding another USD 410.3 million, which constitutes 72.1% of the overall expansion. This period is expected to be characterized by the expansion of specialized testing applications, including synthetic cannabinoid detection panels and prescription medication monitoring tailored for high-risk pregnancies, strategic collaborations between diagnostic laboratories and maternal-fetal medicine programs, and an enhanced focus on universal screening protocols and evidence-based intervention pathways. The growing emphasis on neonatal abstinence syndrome management and rising adoption of umbilical cord tissue testing will drive demand for comprehensive toxicology solutions across diverse neonatal care environments.
Neonatal Toxicology Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 310.2 million |
| Market Forecast Value (2035) | USD 879.6 million |
| Forecast CAGR (2025-2035) | 11.0% |
Why is the Neonatal Toxicology Market Growing?
The neonatal toxicology market grows by enabling neonatal care providers, obstetricians, and child protective services to identify in-utero drug exposure accurately and implement timely interventions while supporting maternal treatment referrals without relying solely on maternal disclosure.
Healthcare professionals and social service agencies face mounting pressure to detect substance-exposed newborns early and initiate appropriate medical management while balancing ethical considerations and legal obligations, with modern neonatal toxicology testing typically providing superior detection sensitivity, extended exposure windows, and comprehensive drug coverage compared to maternal questionnaires alone, making objective screening essential for at-risk infant identification.
The neonatal healthcare industry's need for evidence-based screening protocols and forensic-quality analytical capabilities creates demand for sophisticated toxicology testing solutions that can provide reliable drug detection, ensure legal defensibility, and support clinical decision-making without compromising patient care or maternal confidentiality standards.
Rising substance abuse prevalence and mandatory reporting requirements drive adoption in hospital birthing units, NICU facilities, and reference laboratory networks, where testing accuracy has direct impact on infant safety and intervention effectiveness. The rising opioid crisis affecting pregnant populations creates expanding screening needs for comprehensive opioid panel testing beyond traditional drug categories.
Increasing recognition of neonatal abstinence syndrome and long-term neurodevelopmental impacts enables greater clinical emphasis on early detection and intervention programs. However, cost constraints in resource-limited healthcare systems and ethical debates surrounding universal versus risk-based screening may restrict widespread testing implementation among facilities with limited budgets for comprehensive toxicology panels and concerns about maternal-infant relationship impacts.
Segmental Analysis
The market is segmented by specimen, technology, drug, end use, and region. By specimen, the market is divided into meconium, umbilical cord, urine, and others. Based on technology, the market is categorized into mass spectroscopy and immunoassay.
By drug, the market includes cannabinoids, opioids, cocaine, benzodiazepines, amphetamines, and other illicit drugs. By end use, the market comprises clinical laboratories, hospitals, and others. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Specimen, Which Segment Accounts for the Dominant Market Share?
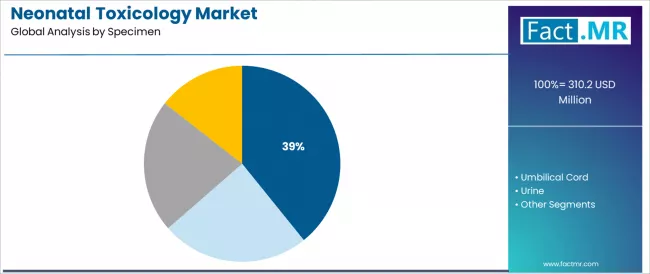
The meconium segment represents the dominant force in the neonatal toxicology market, capturing 39.2% of the total market share in 2025. This established specimen category encompasses solutions featuring extended detection windows and comprehensive drug exposure assessment, including advanced multi-drug screening panels and confirmatory testing protocols that enable superior clinical sensitivity and historical exposure documentation across entire gestational periods and diverse substance categories worldwide.
The meconium segment's market leadership stems from its unique biological characteristics, with specimens capable of accumulating drug metabolites throughout pregnancy, providing retrospective exposure assessment from approximately 20 weeks gestation through delivery while maintaining excellent stability and convenient collection procedures.
Within the meconium category, opioid panels demonstrate particularly strong utilization with 14.3% share of total market, driven by escalating prescription opioid and heroin exposure affecting pregnant populations requiring comprehensive screening for hydrocodone, oxycodone, morphine, and fentanyl compounds. Cannabinoid panels represent another major sub-segment with 12.1% share supporting THC exposure detection, while cocaine/illicit panels account for 7.4% through stimulant and polysubstance abuse screening requirements.
The umbilical cord segment maintains a substantial 32.5% market share, serving neonatal healthcare providers who require practical specimen collection and reliable drug detection for delivery room testing applications. These specimens offer convenient collection procedures performed immediately after birth while providing sufficient detection windows covering the final trimester of pregnancy. The umbilical cord segment demonstrates solid growth potential, driven by simplified collection protocols requiring no specialized training and growing clinical evidence supporting detection equivalency with meconium for many drug classes.
Key analytical advantages driving the meconium segment include:
- Advanced detection window characteristics providing comprehensive gestational exposure assessment covering approximately 20 weeks through delivery enabling thorough maternal substance use evaluation
- Established stability profiles allowing extended storage periods and batch testing optimization without extensive specimen degradation concerns
- Enhanced drug accumulation capabilities enabling detection of intermittent or low-level exposures potentially missed by shorter-window matrices
- Superior clinical utility providing optimal exposure history documentation supporting neonatal abstinence syndrome risk stratification and intervention planning
By Technology, Which Segment Accounts for the Largest Market Share?
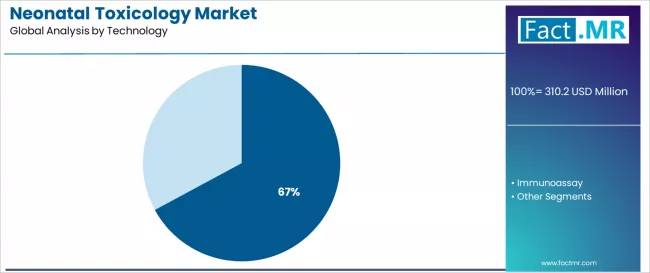
Mass spectroscopy dominates the neonatal toxicology technology landscape with a 67.1% market share in 2025, reflecting the critical role of confirmatory analytical methods in supporting clinical decision-making and legal reporting across neonatal screening programs worldwide. The mass spectroscopy segment's market leadership is reinforced by superior analytical specificity eliminating false-positive concerns, comprehensive compound identification capabilities, and regulatory preferences for definitive testing methodologies in high-stakes clinical and forensic applications.
Within this segment, LC-MS/MS represents the preferred analytical platform with 32.0% share of total market, driven by versatile applications across diverse drug classes including opioids, benzodiazepines, and synthetic compounds requiring high-sensitivity detection and structural confirmation. This sub-segment benefits from established laboratory infrastructure and extensive method validation supporting clinical implementation.
The immunoassay segment represents an important technology category with 32.9% market share, demonstrating continued utilization through cost-effective screening capabilities and rapid turnaround times supporting point-of-care and high-throughput laboratory applications. This segment benefits from simplified operational requirements and established cutoff thresholds enabling efficient sample triage before confirmatory testing.
Key market dynamics supporting technology utilization include:
- Mass spectroscopy expansion driven by regulatory requirements for confirmatory testing and medicolegal documentation demanding analytical certainty
- LC-MS/MS dominance reflecting versatility across compound classes and superior sensitivity enabling comprehensive drug panel detection
- Integration of high-resolution mass spectrometry enabling unknown compound identification and emerging substance detection capabilities
- Immunoassay positioning providing cost-effective preliminary screening supporting efficient laboratory workflows and rapid clinical decision-making
By Drug, Which Segment Accounts for a Significant Market Share?
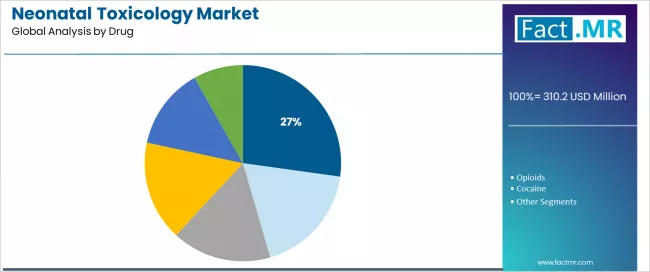
Cannabinoids represent a leading drug category in the neonatal toxicology market with approximately 27.2% market share in 2025, reflecting increasing marijuana legalization impacts and expanding prenatal cannabis utilization requiring comprehensive screening protocols. The cannabinoids segment demonstrates robust demand driven by evolving social acceptance, therapeutic use expansion, and inadequate maternal awareness regarding developmental risks necessitating objective testing programs.
The opioids segment emerges as a critical drug category with 21.4% market share in 2025, driven by devastating opioid epidemic impacts affecting pregnant populations and mandatory screening protocols targeting prescription medication misuse and heroin exposure. Opioid-exposed infants require intensive monitoring for neonatal abstinence syndrome development and comprehensive support services addressing withdrawal management and long-term developmental follow-up.
Within drug categories, cocaine maintains meaningful presence with 16.8% market share through continued stimulant abuse affecting vulnerable populations, while benzodiazepines account for 15.1% supporting prescription medication monitoring including alprazolam and diazepam exposures. Amphetamines represent 11.0% share through methamphetamine epidemic impacts and ADHD medication utilization assessment.
Key drug category dynamics include:
- Cannabinoid testing expansion driven by legalization trends and inadequate maternal risk perception requiring comprehensive THC and metabolite detection
- Opioid screening prioritization reflecting public health crisis and neonatal abstinence syndrome prevention requiring extensive panel coverage
- Polysubstance testing integration addressing complex maternal substance use patterns and comprehensive exposure assessment needs
- Synthetic drug monitoring emphasizing emerging substance detection including synthetic cannabinoids and designer stimulants challenging traditional screening approaches
By End Use, Which Segment Accounts for the Dominant Market Share?
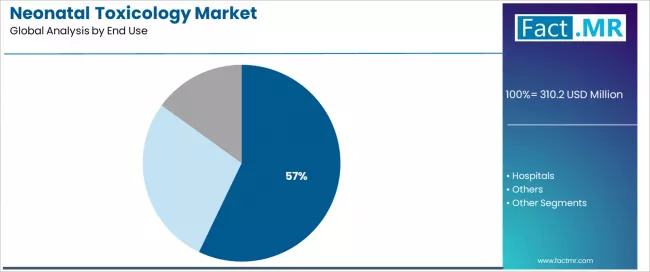
Clinical laboratories represent the leading end-use segment in the neonatal toxicology market with a 57.1% market share in 2025, reflecting the specialized analytical infrastructure and technical expertise requirements for comprehensive neonatal drug testing. The clinical laboratories segment demonstrates consistent demand driven by centralized testing efficiency, comprehensive test menu availability, and established quality assurance programs supporting accurate results and regulatory compliance.
Within laboratory applications, independent reference laboratories demonstrate particularly strong utilization with 31.0% share of total market, reflecting specialized toxicology expertise, advanced instrumentation capabilities, and comprehensive service offerings supporting diverse healthcare facility needs. This sub-segment benefits from economies of scale and dedicated focus on toxicology testing excellence.
The hospitals segment emerges as an important end-use category with 34.9% market share in 2025, driven by point-of-care testing needs and integrated neonatal care delivery requiring immediate screening results for clinical management decisions. Hospital-based testing supports rapid intervention protocols and multidisciplinary care coordination within birthing facilities and NICU environments.
Key end-use dynamics include:
- Clinical laboratory dominance maintaining strength through specialized analytical capabilities and comprehensive quality assurance systems
- Independent laboratory growth driving demand for specialized toxicology services and advanced mass spectrometry platforms
- Hospital integration emphasizing rapid screening capabilities and point-of-care immunoassay implementation supporting immediate clinical decisions
- NICU utilization patterns supporting specialized neonatal testing programs and comprehensive substance exposure assessment protocols
What are the Drivers, Restraints, and Key Trends of the Neonatal Toxicology Market?
The market is driven by three concrete demand factors tied to neonatal health outcomes. First, escalating maternal substance abuse prevalence including opioid crisis impacts creates expanding screening populations requiring comprehensive drug testing, with neonatal exposure representing major public health concern demanding systematic detection approaches. Second, mandatory screening legislation and hospital policy implementation drive testing volume growth, with many jurisdictions establishing universal or risk-based screening protocols supporting child welfare investigations by 2030. Third, technological advances in mass spectrometry sensitivity and specimen collection methods enable more accurate and convenient testing approaches that improve detection capabilities while expanding accessible drug panels and reducing turnaround times.
Market restraints include high testing costs and reimbursement limitations that can challenge healthcare system budgets and universal screening implementation, particularly in resource-constrained settings where comprehensive toxicology panels prove financially prohibitive relative to clinical budgets. Ethical concerns regarding maternal privacy and potential discrimination pose another significant challenge, as neonatal screening without consent raises complex bioethical considerations and may deter prenatal care engagement among substance-using populations, potentially limiting testing acceptance and policy expansion. Specimen collection challenges and result interpretation complexity create additional barriers requiring specialized training, demanding standardized protocols and expert consultation for appropriate clinical utilization.
Key trends indicate accelerated adoption of umbilical cord testing in hospital settings, particularly across North America where convenient collection and reliable performance support widespread implementation preferences over traditional meconium analysis. Point-of-care immunoassay integration trends toward rapid screening capabilities enabling immediate clinical decision-making and streamlined confirmatory testing workflows that optimize laboratory efficiency. However, the market thesis could face disruption if significant shifts in drug legalization policies or major changes in maternal substance abuse treatment paradigms reduce screening emphasis in favor of voluntary treatment engagement and harm reduction approaches.
Analysis of the Neonatal Toxicology Market by Key Countries
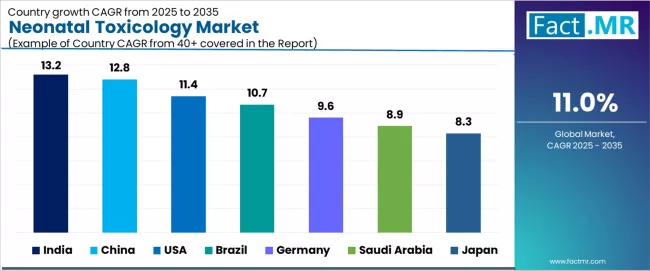
| Country | CAGR (2025-2035) |
|---|---|
| India | 13.2% |
| China | 12.8% |
| USA | 11.4% |
| Brazil | 10.7% |
| Germany | 9.6% |
| Saudi Arabia | 8.9% |
| Japan | 8.3% |
The global neonatal toxicology market is expanding rapidly, with India leading at a 13.2% CAGR through 2035, driven by rising maternal drug exposure and rapid NICU expansion. China follows at 12.8%, supported by increased screening adoption and healthcare reforms. USA records 11.4%, reflecting high prenatal drug use and mandatory screening systems.
Brazil advances at 10.7%, anchored by growing awareness and expanding diagnostic labs. Germany posts 9.6%, focusing on early screening regulations, while Saudi Arabia grows at 8.9%, emphasizing national newborn screening programs. Japan demonstrates 8.3% growth, driven by strong preventive healthcare focus.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the neonatal toxicology market with a CAGR of 13.2% through 2035. The country's leadership position stems from increasing recognition of maternal substance abuse impacts, rapidly expanding NICU infrastructure, and growing healthcare awareness driving adoption of comprehensive newborn screening protocols. Growth is concentrated in major metropolitan areas and tier-1 cities, including Mumbai, Delhi, Bangalore, and Chennai, where multi-specialty hospitals establish advanced neonatal care units offering comprehensive toxicology testing services for at-risk infant populations.
Distribution channels through reference laboratory networks, hospital laboratory partnerships, and specialized toxicology service providers expand testing accessibility across corporate healthcare chains and academic medical centers. The country's rapid NICU expansion provides strong momentum for neonatal testing adoption, including comprehensive training programs supporting healthcare provider education in substance exposure recognition and testing protocol implementation.
Key market factors:
- Rising maternal drug exposure recognition concentrated in urban centers with increasing substance abuse prevalence awareness
- Rapid NICU expansion through private hospital investment and government healthcare infrastructure development initiatives
- Healthcare provider training ecosystem including neonatal care education and toxicology testing protocol implementation
- Laboratory infrastructure growth featuring reference laboratory establishment and advanced analytical capabilities development
Why is China Emerging as a High-Growth Market?
In major healthcare centers including Beijing, Shanghai, Guangzhou, and Chengdu, the adoption of neonatal toxicology testing is accelerating across tertiary hospitals and maternal-child healthcare facilities, driven by healthcare quality improvement initiatives and expanding preventive care emphasis. The market demonstrates strong growth momentum with a CAGR of 12.8% through 2035, linked to increased screening adoption and healthcare reforms supporting comprehensive neonatal care delivery.
Chinese healthcare systems are implementing systematic screening protocols and establishing specialized toxicology testing capabilities to enhance newborn health surveillance while meeting government healthcare quality standards. The country's massive birth population creates substantial testing volume potential, while increasing maternal substance awareness drives clinical emphasis on objective exposure assessment and early intervention.
Healthcare modernization initiatives enable advanced laboratory infrastructure development across provincial maternal-child health centers, establishing comprehensive testing networks supporting both urban and emerging market populations with standardized quality protocols.
Key development areas:
- Increased screening adoption through government healthcare quality initiatives and tertiary hospital protocol implementation
- Healthcare reforms enabling specialized service development and advanced diagnostic capability establishment in maternal-child hospitals
- Laboratory modernization supporting mass spectrometry adoption and comprehensive toxicology panel availability
- Clinical guideline development establishing evidence-based screening protocols and intervention pathways for exposed infants
What drives USA’s Market Resilience?
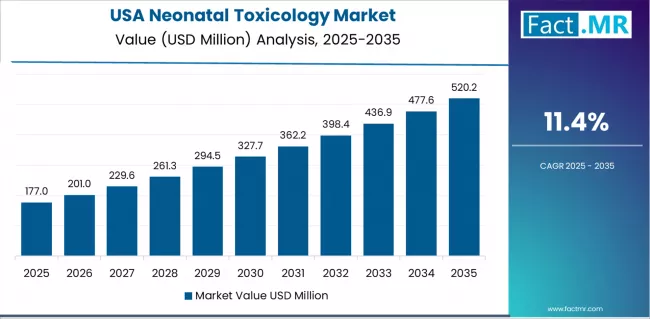
The USA’s market expansion is driven by established mandatory screening legislation, comprehensive child protective services integration, and extensive opioid epidemic impacts requiring systematic neonatal surveillance programs. The country demonstrates steady growth potential with a CAGR of 11.4% through 2035, supported by high prenatal drug use prevalence and mandatory screening systems across many jurisdictions.
American healthcare providers face complex ethical and legal frameworks requiring careful screening policy implementation balancing infant protection with maternal rights considerations. Established testing infrastructure and comprehensive reimbursement support create stable baseline demand for neonatal toxicology, particularly in states with universal screening mandates where hospital policy drives primary testing volume and specialized laboratory utilization patterns.
The expanding integration of maternal substance abuse treatment programs with neonatal testing protocols supports comprehensive care coordination, while advanced mass spectrometry capabilities enable detection of emerging synthetic opioids and designer drugs affecting vulnerable populations.
Market characteristics:
- High prenatal drug use showing substantial affected populations requiring comprehensive screening and intervention services
- Mandatory screening systems implemented across multiple states establishing systematic testing protocols and reporting requirements
- Future projections indicate continued policy expansion with emphasis on universal screening adoption and evidence-based intervention
- Growing emphasis on medication-assisted treatment integration supporting comprehensive maternal-infant dyad care models
How does Brazil show Healthcare Expansion Leadership?
Brazil's neonatal toxicology market demonstrates expanding healthcare awareness focused on substance abuse recognition and diagnostic laboratory capability development. The country maintains solid growth momentum with a CAGR of 10.7% through 2035, driven by growing awareness and expanding diagnostic labs supporting neonatal testing service availability. Major urban centers, including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, showcase advancing toxicology testing adoption where private laboratory networks and tertiary hospitals develop specialized neonatal screening capabilities.
The expanding private healthcare sector enables investment in advanced analytical instrumentation including LC-MS/MS platforms, while public health initiatives promote maternal substance abuse awareness and treatment accessibility. Brazilian medical societies are establishing clinical practice guidelines for neonatal screening, supporting standardized testing protocols and comprehensive intervention pathways that integrate pediatric care with social support services addressing vulnerable mother-infant populations in urban and peri-urban communities.
Key market characteristics:
- Growing awareness supporting maternal substance abuse recognition and neonatal screening emphasis
- Expanding diagnostic labs through private laboratory investment and specialized toxicology service development
- Healthcare provider education initiatives supporting testing protocol implementation and result interpretation guidance
- Public health emphasis driving prevention programs and early intervention service establishment
How does Germany Demonstrate Regulatory Leadership?
The German market leads in systematic newborn screening policy based on integration with comprehensive preventive healthcare frameworks and established child protection protocols. The country shows strong potential with a CAGR of 9.6% through 2035, driven by early screening regulations and structured pediatric care delivery in major regions, including Bavaria, North Rhine-Westphalia, Baden-Württemberg, and Lower Saxony.
German healthcare providers are implementing evidence-based screening guidelines and establishing quality-assured toxicology testing through specialized laboratory networks, particularly within university medical centers and certified pediatric facilities demanding rigorous analytical standards.
Distribution channels through hospital laboratory services and specialized reference laboratories expand coverage across comprehensive maternal-child healthcare systems. The robust regulatory framework ensures laboratory accreditation standards and proficiency testing requirements, while multidisciplinary care teams coordinate obstetric, neonatal, and social services supporting exposed infants and facilitating maternal treatment engagement through integrated care pathways.
Leading market segments:
- Early screening regulations establishing systematic testing protocols and intervention pathway integration
- Quality assurance emphasis supporting laboratory accreditation and analytical method validation requirements
- Multidisciplinary care integration combining obstetric, neonatal, and social service coordination for exposed infants
- Research collaboration supporting evidence generation and screening policy optimization in pediatric populations
What Positions Saudi Arabia for National Program Leadership?
Saudi Arabia's neonatal toxicology market demonstrates expanding screening implementation focused on national newborn screening program development and comprehensive preventive healthcare initiatives. The country maintains solid growth momentum with a CAGR of 8.9% through 2035, driven by national newborn screening programs and government healthcare investment supporting systematic infant health surveillance. Major healthcare centers, including facilities in Riyadh, Jeddah, and Dammam, showcase advancing toxicology testing adoption where national screening protocols integrate substance exposure assessment with comprehensive newborn health evaluation programs.
The Vision 2030 healthcare transformation initiatives support laboratory infrastructure modernization and specialized diagnostic service establishment across regional medical centers. Government investment enables advanced mass spectrometry platform acquisition and technical workforce development, while national screening registries facilitate data collection supporting epidemiological surveillance and intervention effectiveness evaluation across diverse Saudi populations and expatriate communities.
Key market characteristics:
- National newborn screening programs driving systematic testing implementation and comprehensive infant health surveillance
- Government healthcare investment enabling laboratory infrastructure development and specialized service establishment
- Public health emphasis supporting preventive care delivery and early intervention program development
- Healthcare modernization initiatives establishing quality standards and evidence-based screening protocols
How does Japan Show Preventive Healthcare Leadership?
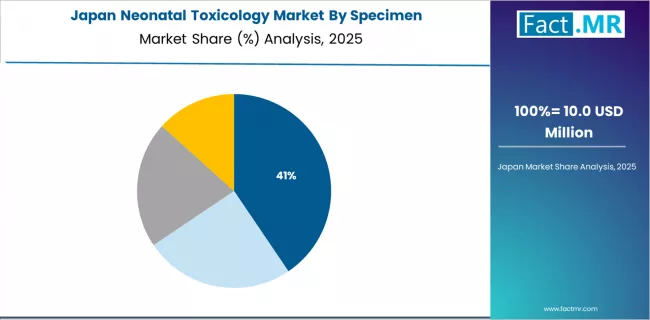
Japan's neonatal toxicology market demonstrates sophisticated preventive healthcare integration focused on comprehensive maternal-child health surveillance and quality-assured laboratory testing. The country maintains steady growth momentum with a CAGR of 8.3% through 2035, driven by strong preventive healthcare focus and systematic infant health monitoring programs.
Major metropolitan areas, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase established neonatal screening capabilities where quality-focused testing protocols integrate with comprehensive pediatric follow-up and maternal support services. The mature healthcare system emphasizes evidence-based screening approaches and rigorous analytical quality standards, with specialized toxicology laboratories maintaining advanced instrumentation capabilities and participating in international proficiency testing programs.
Japanese medical associations provide clinical guidance supporting appropriate screening utilization and result interpretation, while integrated electronic health records facilitate care coordination between obstetric providers, neonatal specialists, and community health services supporting long-term developmental monitoring of exposed infants.
Key market characteristics:
- Strong preventive healthcare focus supporting systematic infant health surveillance and early intervention emphasis
- Quality assurance standards ensuring analytical accuracy and clinical utility in neonatal testing applications
- Maternal-child health integration combining prenatal care with comprehensive newborn screening and follow-up services
- Technology adoption emphasizing advanced analytical methods and reliable toxicology testing capabilities
Competitive Landscape of the Neonatal Toxicology Market
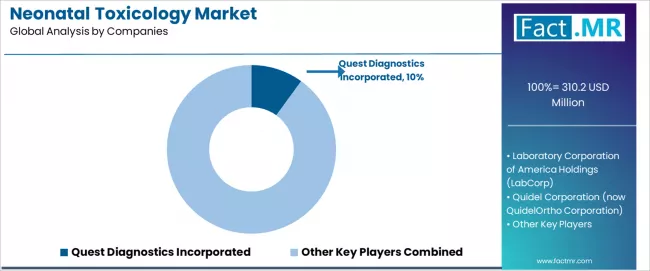
The neonatal toxicology market features approximately 12-15 meaningful players with moderate concentration, where the top three companies control roughly 35-40% of global market share through established analytical capabilities, comprehensive test menus, and extensive hospital partnerships. Competition centers on analytical accuracy, turnaround time, and clinical consultation support rather than price competition alone.
Market leaders include Quest Diagnostics, LabCorp, and Quidel Corporation, which maintain competitive advantages through comprehensive neonatal toxicology portfolios, advanced mass spectrometry platforms, and deep expertise in specialty laboratory services serving hospitals, NICUs, and child protective services, creating high brand recognition among neonatal healthcare providers and strong institutional preferences.
These companies leverage established quality assurance programs and ongoing method validation initiatives to defend market positions while expanding into emerging testing applications including synthetic drug detection and comprehensive medication monitoring.
Challengers encompass Bio-Rad Laboratories and Clinical Reference Laboratory (CRL), which compete through specialized toxicology expertise and innovative testing platforms. Specialized neonatal testing laboratories, including Omega Laboratories, Cordant Health Solutions, and USDTL, focus on dedicated specimen types or comprehensive panels, offering differentiated capabilities in meconium analysis, pain management monitoring, and forensic toxicology applications.
Technology companies and emerging point-of-care developers create competitive pressure through innovative immunoassay platforms and rapid screening technologies, particularly supporting hospital-based testing programs requiring immediate results. Market dynamics favor companies that combine analytical excellence with comprehensive clinical consultation that addresses the complete testing workflow from specimen collection through result interpretation and intervention support.
Strategic emphasis on universal screening protocols, synthetic drug method development, and integrated electronic reporting capabilities enables differentiation in increasingly regulated and clinically focused neonatal toxicology markets across developed and emerging economies.
Global Neonatal Toxicology Market — Stakeholder Contribution Framework
Neonatal toxicology testing represents a critical diagnostic service that enables neonatal healthcare providers, obstetricians, and child protective agencies to identify in-utero drug exposure objectively and implement appropriate interventions while supporting maternal treatment engagement without exclusive reliance on self-reported substance use histories, typically providing superior detection sensitivity, comprehensive drug coverage, and forensically defensible results compared to maternal questionnaires alone while ensuring improved infant safety and maternal support outcomes.
With the market projected to grow from USD 310.2 million in 2025 to USD 879.6 million by 2035 at a 11.0% CAGR, these solutions offer compelling advantages for meconium specimen applications, mass spectroscopy technology platforms, and diverse clinical laboratory settings seeking reliable neonatal screening. Scaling market penetration and ethical implementation requires coordinated action across healthcare policy, screening guidelines, diagnostic laboratories, maternal-fetal medicine programs, and social services coordination.
How Could Governments Spur Local Development and Adoption?
- Screening Policy Development: Implement evidence-based universal or risk-based screening protocols, provide clear guidelines balancing infant protection with maternal rights, and establish standardized reporting requirements supporting child welfare investigations while maintaining appropriate confidentiality.
- Maternal Treatment Infrastructure: Create accessible substance abuse treatment programs for pregnant and postpartum women, establish medication-assisted treatment availability, and support integrated maternal-child dyad care models ensuring family preservation when safe and appropriate.
- Laboratory Quality Standards: Implement certification requirements for neonatal toxicology testing laboratories, establish proficiency testing programs, and create analytical method validation standards ensuring result accuracy and legal defensibility.
- Healthcare Provider Education: Fund training programs for obstetric and neonatal healthcare providers on screening indications, result interpretation, and appropriate intervention, and support multidisciplinary team development coordinating medical and social services.
- Data Surveillance Systems: Establish public health monitoring of neonatal drug exposure trends, support research investigating developmental outcomes, and create evidence bases informing screening policy and intervention effectiveness.
How Could Industry Bodies Support Market Development?
- Clinical Practice Guidelines: Define evidence-based recommendations for screening indications, specimen selection, and confirmatory testing protocols, establish standardized interpretation criteria, and create intervention frameworks supporting clinical decision-making.
- Ethical Standards: Lead discourse addressing maternal-infant rights balance, emphasizing harm reduction and treatment engagement over punitive approaches, and supporting policies maximizing maternal prenatal care engagement.
- Quality Assurance Programs: Develop proficiency testing for neonatal toxicology laboratories, establish method validation standards, and create certification programs ensuring analytical quality and result reliability.
- Professional Education: Run training courses for laboratory personnel, healthcare providers, and social service professionals on optimizing specimen handling, result interpretation, and appropriate intervention in diverse clinical scenarios.
How Could Manufacturers and Technology Players Strengthen the Ecosystem?
- Analytical Innovation: Develop next-generation mass spectrometry methods with enhanced sensitivity, expanded drug panels including synthetic compounds, and simplified workflows that improve detection capabilities while reducing operational complexity.
- Point-of-Care Solutions: Provide rapid immunoassay platforms enabling hospital-based screening with immediate results, facilitate efficient laboratory workflows through preliminary screening, and support timely clinical decision-making.
- Specimen Collection Tools: Offer standardized collection devices, simplified protocols, and quality assurance materials helping healthcare providers obtain optimal specimens and maintain chain-of-custody requirements.
- Clinical Decision Support: Build integrated reporting platforms, evidence-based interpretation guidance, and consultation services helping healthcare providers translate results into appropriate clinical actions.
How Could Healthcare Providers and Laboratories Navigate the Market?
- Evidence-Based Protocols: Implement systematic screening policies based on validated risk assessment or universal testing approaches, establish clear indication criteria, and create comprehensive intervention pathways for positive results.
- Quality Laboratory Services: Partner with accredited laboratories offering validated analytical methods, rapid turnaround times, and clinical consultation support ensuring optimal testing quality and result utility.
- Multidisciplinary Care Teams: Develop integrated approaches combining obstetric care, neonatal medicine, social work, and substance abuse treatment ensuring comprehensive family-centered intervention supporting maternal recovery and infant safety.
- Ethical Practice Standards: Balance mandatory reporting obligations with therapeutic relationships, emphasize treatment engagement over punitive responses, and implement trauma-informed care principles supporting vulnerable families.
How Could Investors and Financial Enablers Unlock Value?
- Laboratory Service Investment: Provide growth capital for established companies like Quest Diagnostics and specialized toxicology laboratories to expand testing capacity, upgrade analytical platforms, and develop comprehensive service offerings supporting market demand.
- Technology Development: Back companies developing advanced mass spectrometry systems, point-of-care screening devices, and digital health platforms that enhance neonatal toxicology capabilities and clinical integration.
- Market Expansion Funding: Finance laboratory network development in high-growth regions including India and China, supporting local partnerships and analytical capability establishment addressing regional testing needs.
- Clinical Integration Solutions: Support companies developing electronic health record integration, clinical decision support tools, and care coordination platforms that optimize neonatal toxicology utilization and intervention effectiveness.
Quest Diagnostics Incorporated
- Laboratory Corporation of America Holdings (LabCorp)
- Quidel Corporation (now QuidelOrtho Corporation)
- Bio-Rad Laboratories, Inc.
- Clinical Reference Laboratory, Inc. (CRL)
- Omega Laboratories, Inc.
- Cordant Health Solutions
- Agilent Technologies, Inc.
- United States Drug Testing Laboratories, Inc. (USDTL)
- Thermo Fisher Scientific Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 310.2 Million |
| Specimen | Meconium, Umbilical Cord, Urine, Others |
| Technology | Mass Spectroscopy, Immunoassay |
| Drug | Cannabinoids, Opioids, Cocaine, Benzodiazepines, Amphetamines, Other Illicit Drugs |
| End Use | Clinical Laboratories, Hospitals, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Brazil, Germany, Saudi Arabia, Japan, and 40+ countries |
| Key Companies Profiled | Quest Diagnostics, LabCorp, Quidel Corporation, Bio-Rad Laboratories, Clinical Reference Laboratory (CRL), Omega Laboratories, Cordant Health Solutions, Agilent Technologies, USDTL, Thermo Fisher Scientific |
| Additional Attributes | Dollar sales by specimen and technology categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with diagnostic laboratories and analytical technology providers, product specifications and analytical requirements, integration with hospital information systems and electronic health records, innovations in mass spectrometry methods and point-of-care screening, and development of specialized applications with synthetic drug detection and comprehensive panel capabilities. |
Neonatal Toxicology Market by Segments
-
Specimen :
- Meconium
- Umbilical Cord
- Urine
- Others
-
Technology :
- Mass Spectroscopy
- Immunoassay
-
Drug :
- Cannabinoids
- Opioids
- Cocaine
- Benzodiazepines
- Amphetamines
- Other Illicit Drugs
-
End Use :
- Clinical Laboratories
- Hospitals
- Others
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Specimen
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Specimen, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Specimen, 2025 to 2035
- Meconium
- Umbilical Cord
- Urine
- Others
- Y to o to Y Growth Trend Analysis By Specimen, 2020 to 2024
- Absolute $ Opportunity Analysis By Specimen, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Technology
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Technology, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Technology, 2025 to 2035
- Mass Spectroscopy
- Immunoassay
- Y to o to Y Growth Trend Analysis By Technology, 2020 to 2024
- Absolute $ Opportunity Analysis By Technology, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Drug
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Drug, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Drug, 2025 to 2035
- Cannabinoids
- Opioids
- Cocaine
- Benzodiazepines
- Amphetamines
- Other Illicit Drugs
- Y to o to Y Growth Trend Analysis By Drug, 2020 to 2024
- Absolute $ Opportunity Analysis By Drug, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Clinical Laboratories
- Hospitals
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Specimen
- By Technology
- By Drug
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Specimen
- By Technology
- By Drug
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Specimen
- By Technology
- By Drug
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Specimen
- By Technology
- By Drug
- By End Use
- Competition Analysis
- Competition Deep Dive
- Quest Diagnostics Incorporated
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Laboratory Corporation of America Holdings (LabCorp)
- Quidel Corporation (now QuidelOrtho Corporation)
- Bio-Rad Laboratories, Inc.
- Clinical Reference Laboratory, Inc. (CRL)
- Omega Laboratories, Inc.
- Cordant Health Solutions
- Agilent Technologies, Inc.
- Value (USD Million)ed States Drug Testing Laboratories, Inc. (USDTL)
- Thermo Fisher Scientific Inc.
- Quest Diagnostics Incorporated
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Specimen, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Drug, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Specimen
- Figure 6: Global Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Technology
- Figure 9: Global Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Drug
- Figure 12: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by End Use
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Specimen
- Figure 29: North America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Technology
- Figure 32: North America Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by Drug
- Figure 35: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by End Use
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Specimen
- Figure 42: Latin America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Technology
- Figure 45: Latin America Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by Drug
- Figure 48: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by End Use
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Specimen
- Figure 55: Western Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Technology
- Figure 58: Western Europe Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by Drug
- Figure 61: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by End Use
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Specimen
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Technology
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by Drug
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Specimen
- Figure 81: East Asia Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Technology
- Figure 84: East Asia Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by Drug
- Figure 87: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by End Use
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Specimen
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Technology
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Drug
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Specimen, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Specimen, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Specimen
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Technology
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Drug, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Drug, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Drug
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the neonatal toxicology market in 2025?
The global neonatal toxicology market is estimated to be valued at USD 310.2 million in 2025.
What will be the size of neonatal toxicology market in 2035?
The market size for the neonatal toxicology market is projected to reach USD 879.6 million by 2035.
How much will be the neonatal toxicology market growth between 2025 and 2035?
The neonatal toxicology market is expected to grow at a 11.0% CAGR between 2025 and 2035.
What are the key product types in the neonatal toxicology market?
The key product types in neonatal toxicology market are meconium, umbilical cord, urine and others.
Which technology segment to contribute significant share in the neonatal toxicology market in 2025?
In terms of technology, mass spectroscopy segment to command 67.1% share in the neonatal toxicology market in 2025.