Specialty Generics Market
Specialty Generics Market Size and Share Forecast Outlook 2025 to 2035
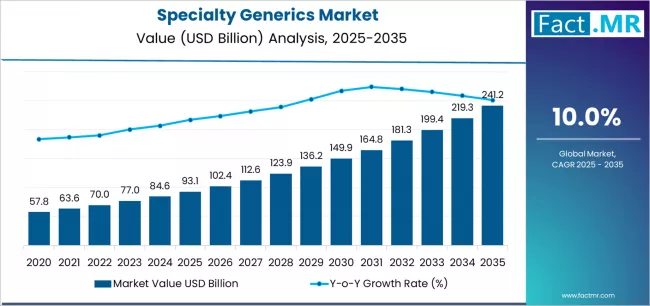
Specialty generics market is projected to grow from USD 93.1 billion in 2025 to USD 241.2 billion by 2035, at a CAGR of 10.0%. Injectables will dominate with a 64.2% market share, while inflammatory conditions will lead the application segment with a 27.5% share.
Specialty Generics Market Forecast and Outlook 2025 to 2035
The global specialty generics market is projected to reach USD 241.2 billion by 2035, recording an absolute increase of USD 148.1 billion over the forecast period. The market is valued at USD 93.1 billion in 2025 and is set to rise at a CAGR of 10.0% during the assessment period.
The market size is expected to grow by approximately 2.6 times during the same period, supported by increasing prevalence of chronic and complex diseases worldwide, driving demand for affordable specialty medications and increasing investments in biosimilar development with advanced manufacturing capabilities across oncology treatments and autoimmune disease therapies globally.
Quick Stats for Specialty Generics Market)
- Specialty Generics Market Value (2025): USD 93.1 billion
- Specialty Generics Market Forecast Value (2035): USD 241.2 billion
- Specialty Generics Market Forecast CAGR: 10.0%
- Leading Type in Specialty Generics Market: Injectables (64.2%)
- Key Growth Regions in Specialty Generics Market: Asia Pacific, North America, and Europe
- Top Players in Specialty Generics Market: Teva Pharmaceuticals Industries Ltd., Viatris Inc., Novartis AG (Sandoz International GmbH), Hikma Pharmaceuticals PLC, Mallinckrodt, Bausch Health Companies Inc., Dr. Reddy's Laboratories Ltd., Endo Pharmaceuticals Inc., Apotex Corp., Sun Pharmaceutical Industries Ltd., Fresenius Kabi Brasil Ltda, STADA Arzneimittel AG
Healthcare systems and patients face mounting pressure to access effective specialty treatments and manage escalating medication costs while addressing patent expiries and healthcare affordability requirements, with modern specialty generic products providing documented therapeutic benefits including bioequivalence to branded specialty drugs, cost savings averaging 30-80% compared to originator products, and expanded patient access to life-saving therapies compared to branded specialty medications alone.
Rising healthcare expenditure pressures and expanding regulatory pathways enabling complex generic approvals create substantial opportunities for pharmaceutical manufacturers and healthcare providers. However, manufacturing complexity and regulatory hurdles may pose obstacles to rapid market entry across technically challenging specialty drug categories.
The injectables segment dominates market activity, driven by extensive clinical application supporting parenteral administration requirements and complex formulation technologies across diverse specialty therapeutic areas worldwide. Healthcare providers increasingly recognize the essential role of injectable specialty generics, with typical product offerings providing hospital-grade administration and controlled delivery systems at competitive pricing through established pharmaceutical distribution networks.
Oral drugs demonstrate steady presence, supported by patient convenience factors and established formulation platforms integrating accessible medication delivery in specialty chronic disease management. Inflammatory conditions emerge as the dominant application segment, reflecting substantial patient populations and established treatment protocols in rheumatology and gastroenterology practice. Specialty pharmacy represents the leading end-use channel, driven by specialized handling requirements and comprehensive patient support services enabling complex medication management.
Regional dynamics show North America maintaining market leadership, supported by high specialty drug utilization rates and established generic substitution frameworks across integrated healthcare systems. Asia Pacific demonstrates the fastest growth trajectory driven by expanding healthcare infrastructure and increasing specialty medication adoption accepting affordable treatment alternatives, while Europe emphasizes biosimilar acceptance and cost-containment policies for sustainable healthcare financing. India leads country-level growth through high unmet medical needs and growing generics adoption, followed by China supported by increasing chronic disease prevalence and government incentives.
The competitive landscape features moderate concentration with Teva Pharmaceuticals Industries maintaining market leadership position at 11.0% share, while specialized players including Viatris, Novartis (Sandoz), Hikma Pharmaceuticals, and Dr. Reddy's Laboratories compete through complex generic portfolios and comprehensive specialty pharmaceutical capabilities across diverse therapeutic applications.
Specialty Generics Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the specialty generics market is projected to expand from USD 93.1 billion to USD 136.9 billion, resulting in a value increase of USD 43.8 billion, which represents 29.6% of the total forecast growth for the period. This phase of development will be shaped by rising demand for biosimilar oncology drugs addressing cancer treatment affordability, product innovation in long-acting injectable formulations with improved patient compliance and extended dosing intervals, as well as expanding integration with specialty pharmacy networks and patient assistance programs. Companies are establishing competitive positions through investment in complex manufacturing capabilities, regulatory filing expertise, and strategic market expansion across hospital systems, specialty distribution channels, and payer contracting agreements.
From 2029 to 2035, the market is forecast to grow from USD 136.9 billion to USD 241.2 billion, adding another USD 104.3 billion, which constitutes 70.4% of the overall expansion. This period is expected to be characterized by the expansion of specialized product applications, including biosimilar immunology portfolios for autoimmune disease management and complex generic inhalation products tailored for specific respiratory conditions, strategic collaborations between generic manufacturers and healthcare systems, and an enhanced focus on value-based contracting models and real-world evidence generation. The growing emphasis on precision medicine accessibility and rising payer preference for cost-effective specialty alternatives addressing budget impact mitigation and therapeutic value optimization will drive demand for comprehensive specialty generic solutions across diverse disease states and patient populations.
Specialty Generics Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 93.1 billion |
| Market Forecast Value (2035) | USD 241.2 billion |
| Forecast CAGR (2025-2035) | 10.0% |
Why is the Specialty Generics Market Growing?
The specialty generics market grows by enabling healthcare systems and patients to access affordable specialty medications while addressing escalating treatment costs and medication affordability without exclusive reliance on high-priced branded specialty drugs.
Healthcare stakeholders face mounting pressure to manage specialty pharmacy budgets and ensure patient access while addressing therapeutic efficacy, safety requirements, and complex handling considerations, with specialty generic products typically providing clinically equivalent therapeutic outcomes achieving bioequivalence to reference products, substantial cost savings ranging from 30-80% below branded pricing, and improved medication adherence through affordability-driven access compared to originator specialty drugs alone, making specialty generics essential for sustainable specialty pharmacy management.
The pharmaceutical industry's need for affordable alternatives to patent-expiring specialty drugs and healthcare system cost-containment imperatives create demand for specialty generic solutions that can provide therapeutic equivalence, enhance patient access, and support formulary optimization without compromising clinical outcomes or medication quality.
Regulatory approval validation and therapeutic equivalence demonstration supporting specialty generic efficacy drive adoption in specialty pharmacy networks, hospital systems, and managed care organizations, where medication costs have direct impact on healthcare budgets and patient affordability.
The expanding global specialty drug spending exceeding USD 400 billion annually with limited generic penetration creates substantial market opportunities for complex generic manufacturers. Rising payer emphasis on specialty tier management and value-based contracting enables informed formulary decisions and adherence to cost-effective medication strategies.
Manufacturing complexity and regulatory pathway uncertainties may limit development timelines and optimal market entry across highly complex specialty molecules serving different therapeutic areas with varying formulation challenges and bioequivalence requirements.
Segmental Analysis
The market is segmented by type, application, end use, and region. By type, the market is divided into injectables, oral drugs, and others. Based on application, the market is categorized into oncology, inflammatory conditions, multiple sclerosis, hepatitis C, and others. By end use, the market includes specialty pharmacy, retail pharmacy, and hospital pharmacy. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Type, Which Segment Accounts for the Dominant Market Share?
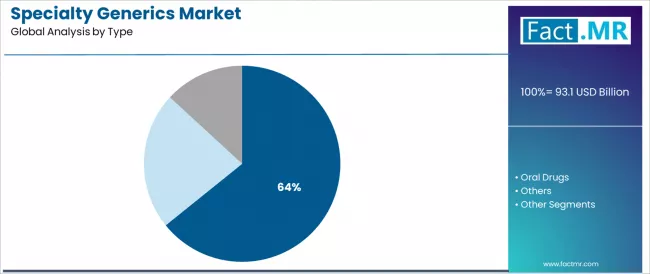
The injectables segment represents the dominant force in the specialty generics market, capturing 64.2% of total market share in 2025. This established type category encompasses solutions featuring parenteral formulations and complex delivery systems, including sterile injectable products for intravenous administration, subcutaneous biologics for chronic disease management, and intramuscular depot formulations that enable controlled drug release and hospital-based specialty treatment across oncology infusion therapy, biologic immunosuppression, and targeted therapeutic interventions worldwide.
The injectables segment's market leadership stems from its critical role in specialty medication delivery, with solutions capable of providing immediate systemic bioavailability bypassing first-pass metabolism, precise dose titration for narrow therapeutic index drugs, and sterile administration supporting immunocompromised patient safety while maintaining manufacturing quality standards and cold-chain distribution integrity across diverse healthcare settings. Within injectables, biosimilar monoclonal antibodies represent rapidly growing applications driven by patent expiries of blockbuster biologic drugs and increasing regulatory acceptance of biosimilar substitution.
The oral drugs segment maintains substantial market presence serving chronic specialty conditions requiring long-term oral medication, addressing patient convenience preferences and home medication management including specialty tablets and capsules for targeted cancer therapies, immunosuppressants for transplant patients, and antiviral medications for chronic infectious diseases.
These solutions offer administration simplicity for patients enabling self-management and medication independence while providing sufficient therapeutic efficacy for appropriate specialty indications. The oral drugs segment demonstrates steady growth driven by expanding oncology oral formulations and specialty neurology medications. The others category includes specialty inhalation products, transdermal systems, and topical formulations serving specific therapeutic niches.
Key clinical advantages driving the injectables segment include:
- Advanced therapeutic delivery mechanisms with demonstrated bioavailability advantages enabling optimal drug exposure and therapeutic response across complex specialty conditions
- Established hospital integration allowing seamless specialty pharmacy coordination and infusion center administration without extensive patient self-management complexity
- Enhanced manufacturing differentiation features requiring specialized aseptic processing, lyophilization technologies, and stability optimization while maintaining regulatory compliance and quality assurance
- Superior market barrier profile providing competitive advantages through technical complexity, capital investment requirements, and regulatory pathway challenges across various specialty injectable categories
By Application, Which Segment Accounts for the Largest Market Share?
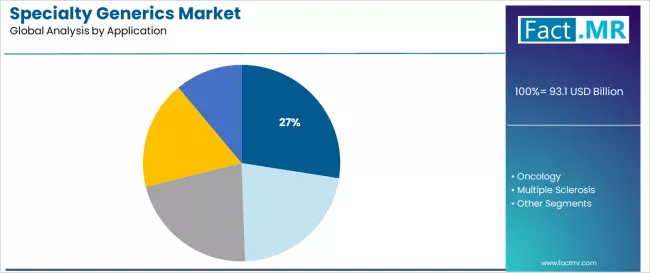
Inflammatory conditions dominate the specialty generics application landscape with 27.47% market share in 2025, reflecting the substantial patient populations and high treatment costs in supporting rheumatoid arthritis, inflammatory bowel disease, and psoriasis management across specialty rheumatology and gastroenterology practices worldwide.
The inflammatory conditions segment's market leadership is reinforced by multiple branded biologic patent expiries including anti-TNF therapies and IL-17 inhibitors, established biosimilar regulatory pathways supporting market entry, and proven clinical equivalence that characterizes successful specialty generic substitution in chronic autoimmune disease management.
Within this segment, rheumatoid arthritis represents the largest patient population, driven by over 18 million global patients requiring biologic therapy and high annual treatment costs exceeding USD 30,000 per patient. This sub-segment benefits from multiple biosimilar approvals and physician acceptance of therapeutic interchange.
The oncology segment represents a rapidly growing application category demonstrating strong expansion through specialized requirements for generic chemotherapy agents, biosimilar supportive care medications, and emerging biosimilar oncology biologics where specialty generics enable affordable cancer treatment access and comprehensive supportive care. This segment benefits from high unmet affordability needs and continuous patent expiries of expensive cancer therapies.
The multiple sclerosis segment maintains important presence through disease-modifying therapy generics serving relapsing-remitting patients, while hepatitis C applications address curative antiviral generic combinations following blockbuster drug patent expiries.
Key market dynamics supporting application growth include:
- Inflammatory conditions expansion driven by biosimilar biologic penetration and cost-containment pressures, requiring proven therapeutic equivalence and physician confidence building
- Oncology advancement trends require specialized handling protocols and comprehensive patient support services for complex cancer therapy generics
- Integration of rare disease generics enabling orphan drug affordability improvement following exclusivity expiration
- Growing emphasis on chronic disease management supporting long-term specialty generic utilization and medication adherence programs
By End Use, Which Segment Accounts for the Dominant Market Share?
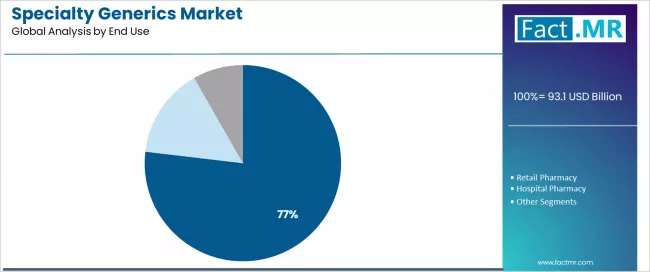
Specialty pharmacy dominates the specialty generics end-use landscape with a 76.87% market share in 2025, reflecting the critical role of specialized distribution and patient support services in supporting complex medication management, prior authorization coordination, and adherence monitoring across specialty therapeutic categories worldwide.
The specialty pharmacy segment's market leadership is reinforced by payer channel preferences for specialty tier management, manufacturer support program integration, and comprehensive patient services that characterize specialty medication distribution for high-cost, high-touch therapies requiring specialized expertise.
Within this segment, integrated specialty pharmacy networks represent dominant distribution models, driven by health system ownership, payer partnerships, and coordinated care delivery. This sub-segment benefits from vertical integration advantages and data-driven patient management capabilities.
The hospital pharmacy segment represents an important end-use category serving inpatient specialty medication administration, acute care specialty interventions, and infusion center operations where hospital-based pharmacists manage complex specialty generics for oncology chemotherapy, immunosuppressive protocols, and specialty antibiotic treatments. This segment benefits from institutional purchasing leverage and clinical pharmacy oversight ensuring appropriate specialty medication utilization.
The retail pharmacy segment maintains limited presence given specialty medication complexity, restricted distribution requirements, and patient support service needs exceeding traditional community pharmacy capabilities.
Key market dynamics supporting end-use growth include:
- Specialty pharmacy dominance driven by complex medication management requirements and comprehensive patient support services, requiring specialized infrastructure and trained personnel
- Hospital pharmacy integration trends require specialty medication formulary management and clinical protocol development supporting evidence-based utilization
- Retail pharmacy limitations reflecting specialty medication distribution restrictions and patient support service requirements beyond traditional dispensing
- Growing emphasis on mail-order specialty pharmacy supporting convenient home delivery and patient adherence monitoring through digital platforms
What are the Drivers, Restraints, and Key Trends of the Specialty Generics Market?
Escalating specialty drug costs and healthcare budget pressures create expanding requirements for affordable alternatives, with specialty generics representing essential cost-containment strategy for payers in comprehensive pharmacy benefit management, requiring accelerated generic approvals.
Increasing chronic disease prevalence and aging populations drive specialty medication utilization growth, with conditions including cancer, autoimmune diseases, and multiple sclerosis demonstrating substantial patient population expansion and corresponding generic substitution opportunities by 2030.
Supportive regulatory frameworks including FDA complex generic guidance and biosimilar approval pathways enable manufacturer investments that improve development feasibility while meeting quality standards and therapeutic equivalence requirements.
Market restraints include manufacturing complexity and technical challenges that can delay generic development and market entry across specialty molecules, particularly when complex formulations require specialized manufacturing capabilities including aseptic processing, biologics production, or modified-release technologies with 5-10 year development timelines.
Regulatory pathway uncertainties and bioequivalence demonstration challenges pose another significant obstacle, as specialty generics require extensive analytical characterization, clinical bridging studies, and real-world safety monitoring, potentially affecting approval timelines and commercial viability.
Physician prescribing inertia and branded product loyalty create additional market penetration barriers, demanding comprehensive medical education initiatives and clinical evidence generation supporting therapeutic interchange confidence. Key trends indicate accelerated biosimilar adoption in developed markets, particularly United States and Europe, where healthcare systems demonstrate increasing acceptance of biosimilar substitution policies enabling automatic interchange, physician education programs, and patient awareness campaigns.
Complex generic innovation trends toward novel delivery systems with extended-release injectables, abuse-deterrent formulations, and patient-friendly administration devices combine improved therapeutic convenience with enhanced medication adherence that optimizes treatment outcomes and patient satisfaction. The market thesis could face disruption if significant patent extension strategies or authorized generic arrangements delay specialty generic entry, or if next-generation targeted therapies rapidly obsolete current specialty medication standards.
Analysis of the Specialty Generics Market by Key Countries
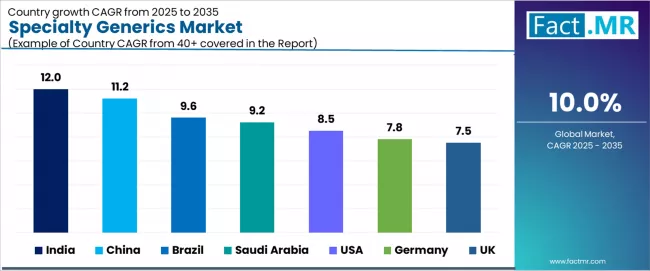
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 12.0% |
| China | 11.2% |
| Brazil | 9.6% |
| Saudi Arabia | 9.2% |
| USA | 8.5% |
| Germany | 7.8% |
| UK | 7.5% |
The global specialty generics market is expanding rapidly, with India leading at a 12.0% CAGR through 2035, driven by high unmet medical needs, growing generics adoption across healthcare system, and healthcare infrastructure expansion. China follows at 11.2%, supported by increasing chronic disease prevalence, government incentives for generic substitution, and expanding urban healthcare access. Brazil records 9.6%, reflecting rising chronic disease prevalence, specialty pharmacy network expansion, and healthcare coverage improvements.
Saudi Arabia advances at 9.2%, leveraging healthcare investment programs, specialty medicine demand growth, and regulatory framework improvements. USA posts 8.5%, focusing on regulatory support initiatives, high specialty pharmacy adoption rates, and large aging population demographics, while Germany grows steadily at 7.8%, emphasizing strong pharmaceutical industry infrastructure and high-quality product demand. UK demonstrates 7.5% growth, anchored by specialty drug access programs, National Health Service adoption policies, and aging population healthcare needs.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the specialty generics market with a CAGR of 12.0% through 2035. The country's leadership position stems from massive unmet medical needs across 1.4 billion population with limited specialty medication access, rapidly growing pharmaceutical manufacturing capabilities establishing India as global generics leader, and expanding healthcare insurance coverage through government schemes including Ayushman Bharat supporting specialty medication affordability.
Growth is concentrated in major metropolitan centers and tier-1 cities, including Mumbai, Delhi, Bangalore, and Hyderabad, where specialty hospitals and corporate healthcare chains are increasingly providing specialty medication access for cancer treatment, organ transplantation, and autoimmune disease management. Distribution channels through hospital pharmacies, specialty medication centers, and emerging specialty pharmacy chains expand product accessibility across urban populations and insured patient segments.
The country's cost-competitive manufacturing infrastructure combined with technical expertise in complex generics provides strong momentum for specialty generic development, including comprehensive capabilities across operations from active pharmaceutical ingredient production through finished dosage formulation and regulatory filing expertise.
Key market factors:
- Manufacturing leadership concentrated in pharmaceutical hubs with established capabilities in complex generic development and biosimilar production
- Healthcare access expansion through insurance coverage growth and specialty hospital infrastructure development
- Comprehensive cost advantages enabling affordable specialty medication production and competitive international export positioning
- Regulatory framework evolution featuring accelerated approval pathways and quality standards alignment supporting generic substitution confidence
Why is China Emerging as a High-Growth Market?
In major metropolitan centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of specialty generic medications is accelerating across public hospitals and specialty treatment centers, driven by rapidly aging population exceeding 280 million elderly citizens and increasing chronic disease burden including cancer, diabetes, and cardiovascular conditions. The market demonstrates strong growth momentum with a CAGR of 11.2% through 2035, linked to comprehensive healthcare reform initiatives and increasing government emphasis on generic medication utilization for cost containment.
Chinese healthcare facilities are implementing specialty generic formularies and volume-based procurement policies to reduce medication costs while serving expanding patient populations requiring specialty treatments. The country's substantial healthcare expenditure growth creates ongoing demand for affordable specialty medications, while government policies including centralized drug procurement and generic substitution mandates drive specialty generic adoption.
Key development areas:
- Healthcare reform acceleration leading generic adoption with emphasis on volume-based procurement and price negotiations
- Chronic disease prevalence growth driving specialty medication demand across oncology, immunology, and metabolic disease categories
- Urban healthcare infrastructure expansion enabling specialty treatment access and complex medication management
- Domestic pharmaceutical industry development supporting local specialty generic manufacturing and reducing import dependence
What Drives USA Market Resilience?
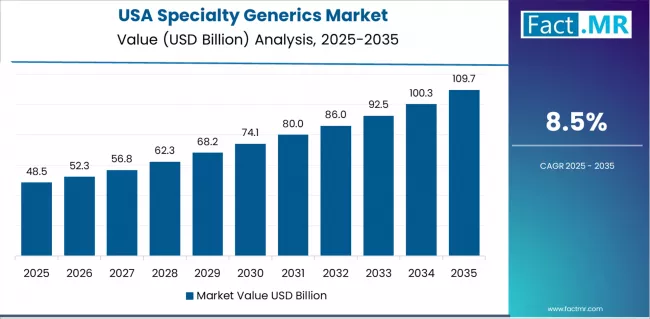
USA market expansion is driven by established specialty pharmacy infrastructure supporting over USD 300 billion annual specialty drug spending, robust regulatory frameworks including FDA biosimilar guidance and complex generic pathways, and substantial aging population with 73 million baby boomers requiring specialty medications. The country demonstrates steady growth potential with a CAGR of 8.5% through 2035, supported by continuous innovation from established generic manufacturers and payer policies incentivizing specialty generic utilization through preferential formulary positioning.
American healthcare stakeholders face cost pressures related to unsustainable specialty drug trend growth and medication affordability challenges, requiring aggressive generic substitution strategies and biosimilar adoption initiatives. However, established specialty pharmacy market with comprehensive patient support services and sophisticated distribution networks create substantial baseline demand for specialty generics, particularly among integrated delivery networks and pharmacy benefit managers where cost-effectiveness and therapeutic equivalence drive formulary inclusion decisions.
Market characteristics:
- Specialty pharmacy maturity and integrated care models showing robust generic adoption with substantial cost savings potential
- Payer policy evolution varying between mandatory generic substitution in some plans and voluntary interchange programs requiring physician approval
- Future projections indicate continued growth with emphasis on biosimilar oncology products and complex autoimmune disease generics
- Growing emphasis on value-based contracting and outcomes-based agreements supporting specialty generic adoption with performance guarantees
How does Germany Demonstrate Pharmaceutical Excellence Leadership?
The market in Germany leads in high-quality specialty generic adoption based on rigorous pharmaceutical standards and comprehensive biosimilar acceptance for enhanced healthcare value. The country shows strong potential with a CAGR of 7.8% through 2035, driven by established pharmaceutical industry infrastructure and healthcare system preferences for cost-effective medications in major regions.
German healthcare providers are implementing specialty generic protocols through evidence-based medicine frameworks and emphasis on therapeutic equivalence validation for comprehensive patient care, particularly in university medical centers and statutory health insurance networks demanding proven clinical credentials. Distribution channels through hospital pharmacies and statutory health insurance pharmacy networks expand coverage across comprehensive healthcare system.
Leading market segments:
- Biosimilar leadership in European markets implementing reference pricing systems and automatic substitution policies
- Quality standards emphasis with rigorous regulatory oversight and post-market surveillance ensuring patient safety
- Strategic focus on rheumatology and gastroenterology biosimilars serving large inflammatory disease populations
- Healthcare system integration supporting specialty generic formulary inclusion and prescriber education initiatives
What Positions UK for Healthcare System Adoption Leadership?
In major healthcare regions including London, Manchester, Birmingham, and Leeds, National Health Service facilities are implementing specialty generic adoption through formulary policies and prescribing guidelines, with documented cost savings showing substantial healthcare budget relief through biosimilar utilization. The market shows steady growth potential with a CAGR of 7.5% through 2035, linked to NHS sustainability initiatives and aging population medication needs requiring cost-effective specialty treatments.
Healthcare providers are adopting specialty generics with NICE guidance to optimize healthcare value while maintaining clinical standards demanded by NHS quality frameworks. The country's established biosimilar acceptance creates ongoing opportunities for specialty generic launches that differentiate through proven clinical equivalence and comprehensive pharmacovigilance.
Market development factors:
- NHS biosimilar adoption policies leading specialty generic utilization with mandatory switching programs in some therapeutic areas
- Aging population demographics driving specialty medication demand across oncology, immunology, and neurology applications
- Healthcare budget pressures supporting aggressive generic substitution and value-based procurement strategies
- Clinical commissioning groups implementing local formularies prioritizing cost-effective specialty medications
How does Brazil Show Emerging Market Leadership?
Brazil's specialty generics market demonstrates developing characteristics focused on expanding chronic disease prevalence and healthcare system modernization aligned with universal health coverage objectives. The country maintains strong growth momentum with a CAGR of 9.6% through 2035, driven by rising cancer incidence, autoimmune disease diagnosis rates, and specialty pharmacy infrastructure development.
Major urban centers, including São Paulo, Rio de Janeiro, and Brasília, showcase growing specialty generic adoption where affordability considerations drive generic prescribing in both public and private healthcare sectors.
Key market characteristics:
- Chronic disease burden growth driving specialty medication demand with emphasis on oncology and immunology therapeutics
- Specialty pharmacy expansion supporting patient access through dedicated distribution channels and support services
- Healthcare coverage improvements enabling specialty medication affordability through public programs and private insurance
- Regulatory framework development supporting biosimilar approvals and generic substitution policies
What characterizes Saudi Arabia's Market Development?
In major healthcare centers including Riyadh, Jeddah, and Dammam, the adoption of specialty generic solutions is expanding across public hospitals and private healthcare facilities, driven by substantial government healthcare investments and Vision 2030 healthcare transformation initiatives. The market demonstrates robust growth potential with a CAGR of 9.2% through 2035, linked to comprehensive healthcare infrastructure development and increasing specialty medication utilization.
Healthcare providers are implementing modern therapeutic protocols and specialty medication access programs supporting chronic disease management and specialty treatment availability. The country's growing healthcare expenditure creates ongoing demand for cost-effective specialty alternatives, while regulatory improvements support specialty generic registrations and market access.
Key development areas:
- Healthcare investment programs leading infrastructure development with emphasis on specialty centers and tertiary hospitals
- Specialty medicine demand growth reflecting epidemiological transition and increasing chronic disease prevalence
- Regulatory improvements supporting accelerated product registrations and quality standards implementation
- Medical tourism positioning requiring access to comprehensive specialty medication portfolios including affordable generic alternatives
Europe Market Split by Country

The specialty generics market in Europe is projected to grow from USD 24.8 billion in 2025 to USD 56.3 billion by 2035, registering a CAGR of 8.6% over the forecast period. Germany is expected to maintain its leadership position with a 28.5% market share in 2025, adjusting to 28.0% by 2035, supported by its dominant pharmaceutical manufacturing infrastructure, comprehensive biosimilar acceptance, and statutory health insurance system supporting generic utilization.
UK follows with a 20.5% share in 2025, projected to reach 21.0% by 2035, driven by NHS formulary policies and aggressive biosimilar adoption programs in major healthcare regions implementing cost-containment strategies. France holds a 18.0% share in 2025, expected to maintain 18.5% by 2035 through ongoing biosimilar uptake and specialty medication access programs.
Italy commands a 16.0% share, while Spain accounts for 12.0% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 5.0% to 5.0% by 2035, attributed to increasing specialty generic adoption in Nordic countries and emerging Eastern European markets implementing biosimilar-friendly policies.
Competitive Landscape of the Specialty Generics Market

The specialty generics market features approximately 20-30 meaningful players with moderate concentration, where the top three companies control roughly 25-30% of global market share through established manufacturing capabilities, comprehensive product portfolios, and extensive distribution networks. Competition centers on complex product development, regulatory expertise, and specialty channel access rather than price competition alone.
Market leaders include Teva Pharmaceuticals Industries with an 11.0% market share, along with Viatris and Novartis (Sandoz), which maintain competitive advantages through vertically integrated specialty generic capabilities spanning complex formulation development through commercial distribution, comprehensive biosimilar portfolios including approved monoclonal antibodies and immunosuppressants, and deep relationships with specialty pharmacy networks and payer organizations, creating high credibility among healthcare stakeholders seeking reliable specialty alternatives.
These companies leverage substantial research and development investments in complex generics and global regulatory filing expertise to defend market positions while expanding into adjacent categories including biosimilar oncology products and specialty inhalation therapies.
Challengers encompass specialized generic manufacturers including Hikma Pharmaceuticals focusing on injectable specialty products, Dr. Reddy's Laboratories emphasizing biosimilar development, and regional players including Fresenius Kabi and STADA Arzneimittel serving specific geographic markets. Diversified pharmaceutical companies including Bausch Health, Mallinckrodt, and Endo Pharmaceuticals maintain specialty generic divisions alongside branded portfolios.
Emerging biosimilar developers and specialty generic startups create competitive pressure through innovative development approaches and targeted product portfolios, particularly in high-value therapeutic areas including oncology biologics and autoimmune disease treatments where first-to-market advantages and clinical differentiation provide commercial opportunities.
Market dynamics favor companies that combine technical manufacturing excellence with comprehensive commercial infrastructure addressing complete market access requirements from regulatory approval through payer contracting, specialty pharmacy partnerships, and patient support program implementation. Strategic emphasis on biosimilar interchangeability designation, real-world evidence generation, and value-based contracting enables differentiation in increasingly competitive specialty pharmaceutical markets across developed and emerging regions.
Global Specialty Generics Market - Stakeholder Contribution Framework
Specialty generics represent a critical pharmaceutical category that enables healthcare systems and patients to access affordable specialty medications while addressing escalating treatment costs and medication affordability without exclusive branded specialty drug dependency, typically providing clinically equivalent therapeutic outcomes achieving bioequivalence, substantial cost savings ranging from 30-80% below branded pricing, and improved medication adherence through affordability-driven access compared to originator specialty drugs alone while ensuring improved patient outcomes and comprehensive healthcare sustainability.
With the market projected to grow from USD 93.1 billion in 2025 to USD 241.2 billion by 2035 at a 10.0% CAGR, these solutions offer compelling advantages for chronic disease management, specialty treatment access, and diverse patient populations seeking effective yet affordable therapies. Scaling market adoption and therapeutic confidence requires coordinated action across regulatory policy, clinical evidence generation, manufacturers, healthcare providers, and payer organizations.
How Could Governments Spur Local Development and Adoption?
- Regulatory Pathway Development: Create clear guidance for complex generic approvals including biosimilars, provide expedited review pathways for first generic applicants, and establish science-based bioequivalence standards supporting innovation while ensuring quality.
- Generic Substitution Policies: Implement automatic substitution laws for specialty generics where appropriate, provide prescriber education about therapeutic equivalence, and establish pharmacist authority for generic interchange supporting adoption.
- Reimbursement Framework: Develop reference pricing systems incentivizing generic utilization, provide favorable formulary positioning for specialty generics, and implement gain-sharing programs rewarding healthcare systems for generic adoption.
- Manufacturing Infrastructure: Fund domestic specialty manufacturing capacity development, provide tax incentives for complex generic production facilities, and support technology transfer programs building local capabilities.
- Market Competition Support: Enforce patent law preventing evergreening strategies, challenge anti-competitive practices restricting generic entry, and promote multiple-source generic competition driving price reduction.
How Could Industry Bodies Support Market Development?
- Quality Standards Development: Define specifications for specialty generic bioequivalence across complex molecules, establish analytical testing protocols and stability requirements, and create quality benchmarking systems supporting manufacturing excellence.
- Therapeutic Interchange Guidelines: Develop clinical guidance for specialty generic substitution, establish safety monitoring protocols and pharmacovigilance requirements, and create prescriber education resources supporting confident switching.
- Biosimilar Acceptance Programs: Lead physician education about biosimilar science, provide patient communication resources addressing safety concerns, and facilitate dialogue between stakeholders building therapeutic interchange confidence.
- Professional Development: Run training programs for pharmacists, physicians, and pharmacy technicians on specialty generic counseling, administration protocols, and patient support service coordination.
How Could Manufacturers and Developers Strengthen the Ecosystem?
- Product Development Excellence: Invest in complex generic development capabilities including aseptic manufacturing, biologics production, and modified-release technologies that enable challenging product launches.
- Clinical Evidence Generation: Conduct real-world effectiveness studies, implement comprehensive pharmacovigilance programs, and generate comparative data supporting therapeutic equivalence claims and building prescriber confidence.
- Market Access Strategies: Develop value-based contracting proposals, provide comprehensive health economics data, and establish patient assistance programs ensuring affordability and access regardless of insurance coverage.
- Specialty Pharmacy Partnerships: Build relationships with specialty pharmacy networks, provide technical training and product education, and establish patient support programs coordinating complex medication management.
How Could Healthcare Providers and Payers Navigate the Market?
- Formulary Management Excellence: Implement evidence-based specialty generic inclusion policies, establish therapeutic interchange protocols with safety monitoring, and develop prescriber education supporting generic adoption.
- Patient Access Programs: Create specialty pharmacy benefit designs incentivizing generic utilization, establish prior authorization policies supporting cost-effective alternatives, and develop patient assistance programs addressing out-of-pocket affordability.
- Clinical Integration: Implement specialty medication management programs coordinating care across providers, establish adherence monitoring systems tracking patient outcomes, and develop quality metrics assessing specialty generic effectiveness.
- Value Assessment: Conduct budget impact analyses comparing specialty generics versus branded alternatives, evaluate total cost of care including medical costs, and negotiate value-based contracts with manufacturers linking payment to outcomes.
How Could Investors and Financial Enablers Unlock Value?
- Manufacturing Investment: Finance specialty generic production facilities, support aseptic manufacturing expansions, and fund biologics manufacturing capabilities enabling complex product development.
- Development Financing: Back companies pursuing challenging generic development programs, support clinical trial execution for biosimilars, and finance regulatory filing expenses for complex approvals.
- Market Expansion Support: Fund geographic expansion strategies, support specialty pharmacy partnerships, and enable commercial infrastructure development including field sales and patient support services.
- Technology Investment: Support digital health platforms improving medication adherence, fund artificial intelligence-driven drug development, and enable data analytics capabilities optimizing market access strategies.
Key Players in the Specialty Generics Market
- Teva Pharmaceuticals Industries Ltd.
- Viatris Inc.
- Novartis AG (Sandoz International GmbH)
- Hikma Pharmaceuticals PLC
- Mallinckrodt
- Bausch Health Companies Inc.
- Dr. Reddy's Laboratories Ltd.
- Endo Pharmaceuticals Inc.
- Apotex Corp.
- Sun Pharmaceutical Industries Ltd.
- Fresenius Kabi Brasil Ltda
- STADA Arzneimittel AG
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 93.1 Billion |
| Type | Injectables, Oral Drugs, Others |
| Application | Oncology, Inflammatory Conditions, Multiple Sclerosis, Hepatitis C, Others |
| End Use | Specialty Pharmacy, Retail Pharmacy, Hospital Pharmacy |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Brazil, Saudi Arabia, and 40+ countries |
| Key Companies Profiled | Teva Pharmaceuticals Industries Ltd., Viatris Inc., Novartis AG (Sandoz International GmbH), Hikma Pharmaceuticals PLC, Mallinckrodt, Bausch Health Companies Inc., Dr. Reddy's Laboratories Ltd., Endo Pharmaceuticals Inc., Apotex Corp., Sun Pharmaceutical Industries Ltd., Fresenius Kabi Brasil Ltda, STADA Arzneimittel AG |
| Additional Attributes | Dollar sales by type and application categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with pharmaceutical manufacturers and generic companies, product specifications and bioequivalence requirements, integration with specialty pharmacy networks and patient support programs, innovations in biosimilar development and complex formulation technologies, and development of specialized applications with therapeutic equivalence demonstration and value-based contracting capabilities. |
Specialty Generics Market by Segments
-
Type :
- Injectables
- Oral Drugs
- Others
-
Application :
- Oncology
- Inflammatory Conditions
- Multiple Sclerosis
- Hepatitis C
- Others
-
End Use :
- Specialty Pharmacy
- Retail Pharmacy
- Hospital Pharmacy
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2025 to 2035
- Injectables
- Oral Drugs
- Others
- Y to o to Y Growth Trend Analysis By Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Inflammatory Conditions
- Oncology
- Multiple Sclerosis
- Hepatitis C
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Specialty Pharmacy
- Retail Pharmacy
- Hospital Pharmacy
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Application
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Application
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Type
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Teva Pharmaceuticals Industries Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Viatris Inc.
- Novartis AG (Sandoz International GmbH)
- Hikma Pharmaceuticals PLC
- Mallinckrodt
- Bausch Health Companies Inc.
- Dr. Reddy's Laboratories Ltd.
- Endo Pharmaceuticals Inc.
- Apotex Corp.
- Sun Pharmaceutical Industries Ltd.
- Fresenius Kabi Brasil Ltda
- STADA Arzneimittel AG
- Teva Pharmaceuticals Industries Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Type
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Type
- Figure 26: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Application
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Application
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Application
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Application
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Application
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the specialty generics market in 2025?
The global specialty generics market is estimated to be valued at USD 93.1 billion in 2025.
What will be the size of specialty generics market in 2035?
The market size for the specialty generics market is projected to reach USD 241.2 billion by 2035.
How much will be the specialty generics market growth between 2025 and 2035?
The specialty generics market is expected to grow at a 10.0% CAGR between 2025 and 2035.
What are the key product types in the specialty generics market?
The key product types in specialty generics market are injectables, oral drugs and others.
Which application segment to contribute significant share in the specialty generics market in 2025?
In terms of application, inflammatory conditions segment to command 27.5% share in the specialty generics market in 2025.